Animation
Aluminium Chloride Molecule
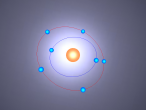
Atoms are the smallest particle of an element and are the basic building blocks of all matter. Atoms are composed of a nucleus which contain positively charged particles called protons and no charged particles called neutrons. Surrounding the nucleus are negatively charged electrons arranged in shells. These electrons exist in a cloud around the nucleus. When two electron clouds of different atoms interact, they bind together to form a molecule. An example of a molecular compound (a molecule that contains atleast two or more different elements) is Aluminium Chloride (AlCl2).
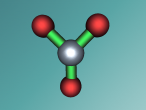
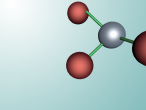
Aluminium Chloride consists of three Chloride atoms and one Aluminium atom which is shown in the image below. These atoms are structured in a trigonal planar with the three chlorine atoms surrounding the single aluminium atom with bonds, 120 degrees apart.The structure of the molecule is based upon temperature change. At temperatures around 190 degrees Celsius Aluminium Chloride converts to Al2Cl6. As temperatures increase further, the molecule is broken up into simple AlCl3 molecules. The aluminium and chloride atoms are bonded covalently, sharing electron pairs in the attractive and repulsive forces between the atoms. However this is only the case for the liquid or vapour form of Aluminium Chloride. When it is in solid form the atoms bond ionically, transferring electrons between atoms.
Aluminium atoms (Al) are composed of 13 protons and 14 neutrons in its nucleus, and 13 electrons surrounding the nucleus. The surrounding electrons are arranged in three shells, 2 in the first, 8 in the second and 3 in the third. Aluminium atoms are one of the lightest atoms with an atomic mass of 26.98 amu. Similarly, Chloride atoms have an atomic mass of 35.45 amu. Chloride atoms have 17 protons and 18 neutrons present in its nucleus and 17 electrons surrounding it in 3 shells. The first shell having 2 electrons, the second having 8 electrons and the third having 7 electrons.
Aluminium Chloride
Compounds are substances which are formed when two or more chemical elements are chemically bonded together. Compounds are found everywhere, from the water (H2O) which we drink, to the aluminium chloride we use as a deodorant. They consist of various atoms arranged in a specific structure, called molecules. Compounds are formed through two types of chemical bonding, covalent and ionic bonding. A covalent bond is formed when electrons are shared between atoms, whereas ionic bonds are formed through the attraction of oppositely (positive charge and negative charge) charged ions.
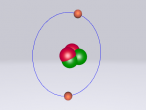
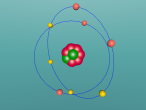
Nitrogen Atom
It is an element that is an essential to all life on Earth, yet in its purest form it can suffocate living organisms. Nitrogen is one of the most important elements on Earth – it can be found in all living systems and makes up around 78% of the Earth’s atmosphere. It is a constituent of protein and nucleic acids. In its gaseous form, nitrogen is colourless, odourless and generally considered inert. In its liquid form nitrogen is also colourless and odourless, and resembles water. Nitrogen was first recognised in 1772 by number of scientists; Carl Wilhelm Scheele, Daniel Rutherford, Henry Cavendish and Joseph Priestley, who all found that air was composed of three different gases, oxygen, carbon dioxide and another gas which they dubbed ‘foul air’ (nitrogen). Nitrogen was first recognised as an element by Antoine Laurent Lavoiser in 1786, and named nitrogen in 1790 by Antoine Laurent Lavoiser. Today, nitrogen is used in food preservation, explosives, and aids medical research.