Technology

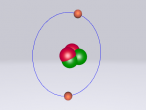
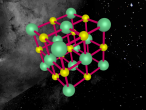
Sodium Chloride Molecule
Atoms are the basic building blocks of matter, fundamental and key to the formation of everything that exists around us. They make up the air, our bodies and even the very screen that you are staring at right now. Each atom has its own unique atomic structure along with its different characteristics.
These atoms join together into groups to form molecules of either elements or compounds. Elements can only consist of the same type of atoms that cannot be broken down into simpler substances, whereas compounds are made of two or more elements that are chemically bound together. An example of an important molecule in everyday life is Sodium Chloride or commonly known as salt.
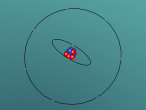
3D Model of Fluorine Atom
3D Models of Atom Blog – Fluorine
Introduction:
The topic of this blog is going to be about an atom i.e. fluorine. Using a program called VRMath2.0, my group created a 3D model of fluorine which included the nucleus of the atom and its outer shells. This blog will include the following components: a 3D model of the atom Fluorine, a description on the composition, structure and characteristics of Fluorine, questions that I wish to investigate further and the difficulties that I experienced throughout the programming.
Fluorine is a chemical element represented by the symbol ‘F’ and has an atomic number of 9. It is classified as a non-metal and is the first element in the family of halogen gases. Given that it is the top element in the Halogen Group, it is the most electronegative element and therefore is very reactive. (Feldman, R. & Marsden, S., 2015) There are a few common usage of Fluorine and this element can be found in a variety of items and objects including rocket fuel, refrigeration fluids and toothpaste (helps to prevent tooth decay). (Chem4Kids, 2016)
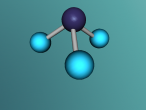
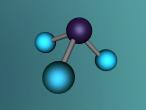
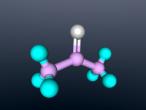
Ammonia molecule
Atoms are the basic building blocks of all matter. This means that everything around you, including yourself, are made up of atoms. However, what happens when these atoms join together chemically? Well, they form compounds called molecules. Molecules are composed of chemically-bonded atoms and they have a neutral charge unlike an ion, meaning that they have the same amount of protons and neutrons total. Atoms can never be created nor destroyed during a chemical reaction, so they can only break off and form new molecules. Ammonia is a type of molecule and is composed of of one nitrogen atom and three hydrogen molecules, with the chemical equation being NH3. It is described as a colourless, pungent gas. The major use of ammonia is in fertilisers because they are a great source of nitrogen in fertilisers (nitrogen enhances leaf growth). The chief commercial method of producing ammonia is by the Haber-Bosch process, involving the direct reaction of elemental hydrogen and elemental nitrogen. Its boiling point is -33.35oC and its freezing point is -77.7oC.
Ammonia Molecule
Ammonia (NH3) is a molecule which consists of three hydrogen atoms and a nitrogen atom bonded together. It is a colourless, odorous, alkaline gas which is produced when organic materials decompose. This molecule is essential to many plants and animals, as a source of nitrogen. Additionally, bacteria within the intestines can produce the substance. Ammonia has many uses, though it is mostly used in fertilisers and cleaning products.
The Potassium Atom
Potassium is a chemical element classified the atomic symbol 'K' on the Periodic Table. As the seventh most abundant element on Earth (2.6% abundance in Earth's crust), potassium is found in a wide array of industries, despite being commonly known for its integral role as a mineral that allows human cell function and its presence in bananas. Some applications of potassium are (but not limited to) potassium-based fertilisers, pharmaceuticals and manufacturing. Discovered in 1807 by Sir Humphrey Davy, its abundance and simple atomic structure has allowed for exhaustive research and application regarding potassium.