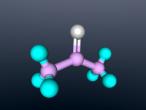
Acetone
Atoms are the basic building blocks of matter; they form everything around us. These building blocks join to form elements and compounds. Elements consist of the same type of atoms and can not be disarranged, whereas compounds are comprised of two or more types of atoms and can be separated into simpler elements physically and chemically. All compounds are molecules, which are formed when two or more atoms are bonded chemically. An example of a molecule is acetone, a chemical which is found very commonly in our daily lives.
Acetone, also known as propanone, is used in the production of chemicals as it possesses useful properties. Because it is a solvent, it is commonly manufactured and included in many cleaning products, such as nail polish, paint and adhesive removers. Acetone is also found in landfills, tobacco smoke, volcanic gases, plastics, pharmaceutical products. The chemical is also a byproduct of metabolic processes in plants and animals, including humans.
The molecular formula for acetone is C3H6O; there is one oxygen atom, 3 carbon atoms and 6 hydrogen atoms. Acetone molecules are unbalanced, contributing to their polarity. The oxygen consists of two unshared electron pairs, causing one end of the molecule to be negative. However, the two carbons atoms which are each singularly bonded with with three hydrogen atoms have positive charges. Due to the disproportionate distribution of charges throughout the molecule, it is polar.
The white ball represents the oxygen atom, the pink balls represent carbon and the blue balls, hydrogen. The cylinders in between are the bonds.
The oxygen atom is double bonded with a central carbon, which is then bonded to two hydrocarbon groups. The central carbon shares bonds with the other two carbons and these two carbons each share bonds with three hydrogen atoms. Because acetone contains a carbonyl group joined to two hydrocarbon groups, it is a ketone. The bonds between the carbon and hydrogen are very covalent whilst the oxygen and central carbon have a moderately covalent bond. Covalent bonds are molecular bonds where atoms share electron pairs, ensuring the stability of attractive and repulsive forces.
Acetone is a clear liquid and is often mistaken for other liquids such as water and alcohol. However, it can be distinguished by its pungent odour and taste. Acetone is volatile, thus, it evaporates at relatively low temperatures. It has a boiling point of 56.2°C and a freezing point of -94.8°C. In liquid form, its density is 0.78440 g/mL at 25°C and it has viscosity of 0.32 cP at 20° C. Acetone is also miscible with water, so it forms a homogeneous mixture when combined. Another characteristic of acetone includes its fluorescence under ultraviolet light, allowing it to be a successful tracer fluid in procedures such as fluid flow experiments.
My partner and I programmed separately and then decided on which 3D model to use. During programming, I found it was difficult to position the turtle in the correct location so that the hydrogen molecules and the bonds they share with the carbon were at the same angle. Thus, the molecules' measurements were not precise. I was also very unfamiliar with the the programming codes which made it even more challenging. Despite this, I find the concept of using the turtle to place objects clever.Overall, the excursion was a very beneficial learning experience for me and allowed me to expand my knowledge about molecules and programming, in addition to being enjoyable.
Below are a few links I have sourced information from:
Here are the codes used to program the 3D model:
CLEAN HOME RESET
setbg 33
; carbon in middle
setmat 10 31 ball
transform setparent object
transform setparent object
ru 90 fd .5 west .15 rd 90
setscale .25 .75 .25 can
east .3 can
ru 90 fd .75 rd 90
setmat 5 30 can
west .3 can
ru 90 fd .5 east .15
;oxygen
setscale 1 1 1 ball
;back at proper orientation middle carbon
bk 1.75 rd 90
rt 90 rd 20 fd 1 rd 90 setscale .5 1.25 .5 setmat 10 31 can
ru 90 fd 1
;carbon on right
setscale 1 1 1 ball ru 80
fd .5 rd 90 setscale .5 .2 .5 can
ru 90 fd .2 rd 90 setmat 10 18 can
;hydrogen above right
ru 90 fd .5 setscale 1 1 1 ball
bk 1.2 rd 80 rt 60 rd 30
fd .5 rd 90 setscale .5 .2 .5 setmat 10 31 can
ru 90 fd .2 rd 90 setmat 10 18 can
ru 90 fd .5
;hydrogen front right
setscale 1 1 1 ball
bk 1.2 ru 30 rt 240
rd 30
fd .5 rd 90 setscale .5 .2 .5 setmat 10 31 can
ru 90 fd .2 rd 90 setmat 10 18 can
ru 90 fd .5
;hydrogen back right
setscale 1 1 1 ball
bk 1.2 ru 30 rt 240 fd 2 rd 20
rd 20 fd 1 rd 90 setscale .5 1.25 .5 setmat 10 31 can
ru 90 fd 1
;carbon on left
setscale 1 1 1 ball ru 80
fd .5 rd 90 setscale .5 .2 .5 can
ru 90 fd .2 rd 90 setmat 10 18 can
;hydrogen above left
ru 90 fd .5 setscale 1 1 1 ball
bk 1.2 rd 80 rt 60 rd 30
fd .5 rd 90 setscale .5 .2 .5 setmat 10 31 can
ru 90 fd .2 rd 90 setmat 10 18 can
ru 90 fd .5
;hydrogen back left
setscale 1 1 1 ball
bk 1.2 ru 30 rt 240
rd 30
fd .5 rd 90 setscale .5 .2 .5 setmat 10 31 can
ru 90 fd .2 rd 90 setmat 10 18 can
ru 90 fd .5
;hydrogen front left
setscale 1 1 1 ball
bk 1.2 ru 30 rt 240 fd 2 rd 20SPIN "obj_1 "LT 7
Feel free to leave questions in the comments.