Hydrochloric Acid (HCl)
Hydrochloric Acid
by Felix Tang
Atoms, Substances, Elements, Compounds, Mixtures - What are they?
The Earth is made up of trillions upon trillions of substances. Metals, rocks, liquids, gases, acids, solids, every element on Earth and in the Universe. They are all made up of atoms, the basic building blocks of matter and which form everything in the Universe. Each atom has its specific properties, such as atomic mass, atomic number, stability, and specific isotope. When in the proper conditions, they may chemically bond and form crystal lattices to form different elements or compounds. When different, these atoms may bond to each other to create different substances.
A substance made entirely out of one atom is called an Element, and is pure, for example the gas Oxygen. A substance made up of 2 or more different atoms chemically bonded to each other is called a compound, or a molecule. An element may not be broken down to a simpler form using chemical or physical methods. However, a compound may do so chemically because there are different types of atoms comprising it. The combination of 2 or more compounds or elements creates a mixture, which can be reverted to its original states chemically or physically. An important type of molecule secreted in the human body and is vital for our disgestion is Hydrochloric Acid, or HCl.
What is Hydrochloric Acid?
Hydrochloric acid is a clear, colourless and highly pungent acid that is secreted naturally in the stomach during digestion and forms a major part of gastric acid. It is the most potent acid secreted by humans and digests all food that reaches the stomach. In its natural state, Hydrochloric Acid is a colourness and poisonous gas due to containing chlorine gas, which is highly toxic to the human body. However, it is mostly dissolved in water to become its liquid form.
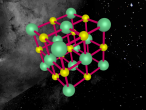
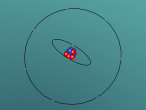
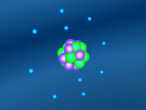
Its chemical formula is formed from a Hydrogen atom binded to a Chlorine atom (HCl). Chlorine is the 17th element on the periodic table, and has an atomic number of 17, meaning it has 17 protons inside its nucleus, and therefore 17 electrons orbiting the atom. Hydrogen is the first element on the periodic table, and therefore is the smallest and contains the least amount of particles. In fact, it contains only 1 proton in the nucleus and 1 electron. When the two atoms bind together, the hydrogen's electron allows the Hydrochloric Acid molecule to complete its 3 atom shells of 2-8-8.
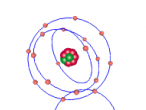
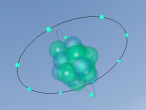
Below is a model of a Hydrogen atom:
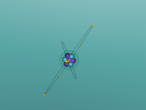
A model of a Chlorine atom:
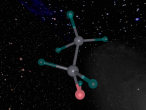
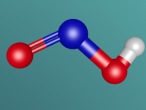
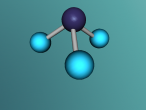
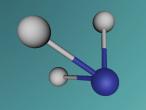
And a model of a hydrochloric acid molecule:
As shown in the model of the hydrochloric acid molecule, the Hydrogen atom is covalently bound to the Chlorine atom. Covalent bonds are chemical molecular bonds between atoms which involve the sharing of electron pairs between them. Covalent bonds help to keep a stable balance of attractive and repulsive forces between atoms due to the balance of positive and negative attractions.
How is Hydrochloric Acid unique from other acids?
Hydrochloric acid is a unique acid as it is secreted by the human body and is an important acid both for digestion and in commercial use. Therefore, it has several defining physical and chemical properties. It is clear, colourless, has a strong, pungent odour and is a strong acid, which is why vomit is often pungent in addition to the regurgitated food, and has an acidic taste. Because of the acidity of hydrochloric acid, the mouth also secretes lots of saliva to protect the mouth prior to vomiting.
What are the physical and chemical properties of Hydrochloric Acid?
At a 38% solution, its melting point is −26 °C, and its boiling point is 48°C. Its molar mass is 36.46g/mol and its density is 1.18g/cm³ at 37% solution. It also has a viscosity of 1.9 mPa·s at 25°C and at a 31.5% solution. It is also fully miscible, which means it is able to homogeneously mix with water to form a uniform texture and properties. When it is dissolved in water, it releases many hydrogen ions. Ionisation occurs when acids are dissolved in water, and an atom loses or gains electrons. Hydrogen atoms lose electrons (becoming positively charged) and Chlorine atoms gain electrons (becoming negatively charged). A stronger acid releases many more electrons than weaker acids in water. Due to this ability, hydrochloric acid is classified as one of the 6 strong acids as it is able to essentially disassociate 100% in solutions of 1.0 Molar and less. For example, hydrochloric acid releases more electrons in water than Vinegar or Lemon Juice.
Due to the high corrosiveness of hytdrochloric acid, it is unsuitable for storage in metal containers and must be stored in plastic containers to avoid ruining the container.
When researching and constructing the 3D model of hydrochloric acid, the Hydrogen atom was undoubtedly the easiest to create as it was the smallest atom and contained only 1 electron and 1 proton. However, the process of creating the model of the Chlorine atom was harder due to the 17 electrons, protons and neutrons in the nucleus of the atom, and our general inexperience with the vrmath program and the logo programming language produced difficulties at first. As a result, my partner and I were advised by several helpers and teachers on the appropiate processes and logic of the molecule, and through the process of experience and trial and error we were able to successfully create the full hydrochloric acid molecule, showing the binding of the hydrogen and chlorine atoms.
The investigation overall helped me understand more about acids and their properties, not just of hydrochloric acid's, and that most acids are in their natural form a gas. I also learned a lot about the industrial and commercial applications of both strong and weak acids, and the differences between different types of acids.
Some questions I would like to investigate further are:
Can hydrochloric acid be found naturally outside a multicellular organism?
How did the production of hydrochloric acid become available to the human stomach?
Why does the stomach not produce more powerful or corrosive acids?
Can hydrochloric acid bond with other elements or compounds to create more or less corrosive substances?
Listed below are links that provided helpful information in the research and writing of this blog post and model creation.
http://chemistry.about.com/od/acidsbase1/a/strong-acids-list.htm
http://chemistry.elmhurst.edu/vchembook/106Amixture.html
https://www.britannica.com/science/hydrochloric-acid
http://blog.polyprocessing.com/blog/industrial-uses-of-hydrochloric-acid-storage-concerns
http://www.newworldencyclopedia.org/entry/Hydrochloric_acid
The programming for the Hydrogen model:
The programming for the Hydrochloric Acid model: