Nitrous Acid
Nitrous Acid
Chemistry is a broad field of physical science dealing with the composition and property of elementary substances that make up matter and the world around us. During the chemistry workshop at QUT, my partner and I have completed a 3D model of the molecule nitrous acid by programming through the VRMath 2.0 website. This blog will provide a variety of information on the molecule we have chosen, ranging from its utility to the scientific explanation of the molecule’s structure.
Nitrous acid is a weak and unstable acid only present as aqueous solutions and nitrate salts. It can be produced naturally through the combination of nitric oxide (NO) and water (H2O), by manually dissolving dinitrogen trioxide (N2O3) in water or by reacting sodium nitrate (NaNO2) with mineral acids. The acid helps to regulate the ozone content within the Earth’s atmosphere. It is also very useful in industry, prominently used to prepare diazonium salts before combining it with anilines and phenols to make azo-dyes. Additionally, it the acid utilized to destroy sodium azide solutions, which could potentially pose toxic and explosive threats to the surrounding environment and people.
Typically pale-blue in colour, nitrous acid has a mass of 47.013g/mol with a density of 1g/mL. In its gas phase, the molecule is able to exist as either a trans or cis isomers. The molecule itself is not severely toxic, but it may affect the respiratory system and stir up irritation symptoms. The acid is a powerful oxidiser, and direct contact with phosphorus trichloride (PCl3) will cause explosions. To this date, both the melting and the boiling point of the molecule are still unknown.
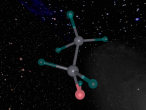
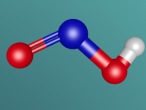
Nitrous acid, HNO2, is composed of 4 atoms, 3 of which are of different elements, therefore making it a compound. The molecule is structured to have one nitrogen atom (N) attached to two electro-negative oxygen atoms (O) in a large ‘v’ shape. One of the oxygen atoms has a smaller hydrogen atom (H) connected to it. The other oxygen atom is connected to the nitrogen atom through a double covalent bond. This has occurred due to the atoms sharing two pairs of electrons as opposed to one pair. By doing this, the molecule can retain a more stable structure.
Below are some links for further information on nitrous acid:
http://www.newworldencyclopedia.org/entry/Nitrous_acid
http://www.softschools.com/formulas/chemistry/nitrous_acid_uses_properties_structure_formula/232/
https://pubchem.ncbi.nlm.nih.gov/compound/nitrous_acid
As there was a limited amount of accessible information on nitrous acid, I would like to be able to investigate further on the effects temperature can hold on the stability of the molecule and its bonds. I would also like answers on the initial discovery and utilisation of nitrous acid.
During this process of constructing a 3D model using the VRMath 2.0 program, I have encountered many problems, especially with correctly applying the commands at to achieve what I wanted to. This had slowed me and my partner’s progress down considerably. Since both of us had never really been engaged in such intense programming before, it started out quite difficult to complete tasks, but as time went on it started to become much easier. Additionally, both my partner and I had troubles saving work that was still in progress, so it led to a lot of frustration in restarting our whole project over and over again; however, we had still managed to complete our 3D model at the end, which caused a great deal of satisfaction. Overall though, it was a great opportunity to learn and begin to grasp a whole new concept that was quite unfamiliar to me before.
By: Sunny Fan