Animation

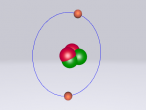
Sodium Chloride Molecule
Introduction
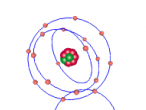
Atoms are the basic building blocks of matter, fundamental and key to the formation of everything that exists around us. They make up the air, our bodies and even the very screen that you are staring at right now. Each atom has its own unique atomic structure along with its different characteristics.
These atoms join together into groups to form molecules of either elements or compounds. Elements can only consist of the same type of atoms that cannot be broken down into simpler substances, whereas compounds are made of two or more elements that are chemically bound together. An example of an important molecule in everyday life is Sodium Chloride or commonly known as salt.
Sulphuric Acid
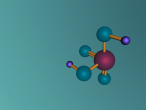
Sulphuric acid is a pungent-ethereal, colourless to slightly yellow viscous liquid that is soluble in water at all concentrations. It is highly corrosive, dense and oily and one of the most important of all chemical, prepared industrially by the reaction of water (H2O) with sulphur trioxide (SO3). In different concentrations, the acid is used to manufacture fertilisers, pigments, dyes and detergents as well as in petroleum refining and metallurgical processes. Its most common use is in lead-acid storage batteries. In this blog, there will be a detailed analysis of the use of sulphuric acid, its bonds and characteristics.
Sulphuric Acid
Sulphuric acid is a pungent-ethereal, colourless to slightly yellow viscous liquid that is soluble in water at all concentrations. It is highly corrosive, dense and oily and one of the most important of all chemical, prepared industrially by the reaction of water (H2O) with sulphur trioxide (SO3). In different concentrations, the acid is used to manufacture fertilisers, pigments, dyes and detergents as well as in petroleum refining and metallurgical processes. Its most common use is in lead-acid storage batteries. In this blog, there will be a detailed analysis of the use of sulphuric acid, its bonds and characteristics.



















 I
I