Sulphuric Acid
Sulphuric acid is a pungent-ethereal, colourless to slightly yellow viscous liquid that is soluble in water at all concentrations. It is highly corrosive, dense and oily and one of the most important of all chemical, prepared industrially by the reaction of water (H2O) with sulphur trioxide (SO3). In different concentrations, the acid is used to manufacture fertilisers, pigments, dyes and detergents as well as in petroleum refining and metallurgical processes. Its most common use is in lead-acid storage batteries. In this blog, there will be a detailed analysis of the use of sulphuric acid, its bonds and characteristics.
Sulphur is composed of Sulphur, oxygen and hydrogen. Sulphur is found in the oxygen family and is considered among the main group elements. It has an atomic number of 16 and an atomic mass of 32.06 grams. It has a density of 2.07 g/cubic centimetre and has a melting and boiling point of 115 and 441 degrees Celsius respectively. Oxygen belongs to the oxygen family and has an atomic number of 6. It has an atomic mass of 15.999 grams with a density of 0.001308 g/cubic centimetre. It has very low boiling and melting points. Hydrogen is the last element which makes up sulphuric acid. It has an atomic number of 1 and very low melting and boiling points. It is also very light and has a density of 0.000082 grams/cubic centimetre with an atomic mass of 1.008. The combination of all these elements make sulphuric acid. Because of the composition of these elements and there are different types of bonds which make up this compound.
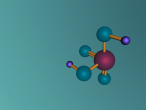
Sulphuric acid contains a distinct ionic bond between H+ and HSO4-. Ionic bonding is when atoms either transfer or share their valence electrons. In the case where one or more atoms lose electrons and other atoms gain them in order to produce a noble gas electron configuration, the bond is called an ionic bond. Since sulphuric acid is a strong acid, so the two ions are dissociated in water. Sulphur also double bonds with two of the oxygen molecules, forming a covalent bond. Covalent bond involves the sharing of a part of electrons by two atoms, in contrast to the trainer of electron in ionic bonds. Not only are these bonds either covalent and ionic but also they are at a certain angle. Sulphuric Acid forms a shape of a tetrahedral with the bonds at a degree of 109.5.
Sulfuric acid is a colorless oily liquid. It is soluble in water with release of heat. It is corrosive to metals and tissue. It will char wood and most other organic matter on contact, but is unlikely to cause a fire. Density 15 lb / gal. Long term exposure to low concentrations or short term exposure to high concentrations can result in adverse health effects from inhalation. It has a specific gravity of 1.84. It has a melting point of 10 degrees Celsius and a boiling point of 348 degrees Celsius.
I wanted to look at how and why there are different types of bonding in sulphuric acid (ie. Both covalent and ionic) and I therefore would go to the following websites:
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond.html
https://pubchem.ncbi.nlm.nih.gov/compound/sulfuric_acid#section=Top
http://www.npi.gov.au/resource/sulfuric-acid
http://www.ucc.ie/academic/chem/dolchem/html/comp/h2so4.html
http://www.business-science-articles.com/matric/chem/358-physical-properties-of-sulphuric-acid-chemical-properties-of-h2so4
There were many difficulties which I had encountered on the way. It is an interesting way of generating a atom/molecule in a new fashion; computer coding. I throughly enjoyed the VRMath 2.0 workshop because it was a different experience into an unfamiliar field. The main difficulty I had faced was that it was a nuisance if I just forgot to add a bracket or semicolon, it would cause the whole coding to not work. This means that I had to go through the code again, in the end just to find that I spelt 'down' wrong! Although it was a tedious process, when I had finally completed the task, I was deeply satisfied with the end result.
Groups:























Comments
good analysis. You cld have
good analysis. You cld have reflected on more qsns and use in-text