Phosphorus blog

Introduction:
The following post will be about the element phosphorus and its various properties and uses. It is a very complex atom, with a large number of electrons, protons, and neutrons making it up. Its symbol on the periodic table is P and has an atomic mass of 15. It is found in two main forms: White phosphorus and red phosphorus. It is not found free in nature because it is a highly reactive element.
Information:
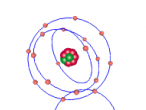
Phosphorus is composed of fifteen protons and sixteen neutrons, with fifteen electrons orbiting around the nucleus where these are located. The first form of phosphorus found was white phosphorus in 1669. Phosphorus emits a faint glow when exposed to oxygen, hence it being named after the Greek word for 'light-bearer'. The term phosphorescence comes from this element, as it means 'to glow'. The process that phosphorus goes through to glow is called 'chemiluminescence'.
Life is impossible without phosphorus. It contributes vital parts to DNA, RNA, ATP and the phospholipids, which form all cell membranes. Human urine and bone ash were both early sources of this element. Phosphorus also can act as a fertiliser for aquatic environments. There are two main types of phosphorus: white and red.
White phosphorus is the most useful of the two, and is the most volatile and least dense of all kinds. Over time, white phosphorus turns into red phosphorus, a process that may be accelerated by light and heat. White phosphorus is extremely toxic, and ingestion should be avoided, unless sever liver damage is wanted. Red phosphorus is the more stable of the two.
Further Reading:
Oregon State University:
http://lpi.oregonstate.edu/mic/minerals/phosphorus
National Kidney Foundation:
https://www.kidney.org/atoz/content/phosphorus
Jefferson Lab:
http://education.jlab.org/itselemental/ele015.html
Mathematics & Programming:
Not only has this task allowed me to learn more about the element phosphorus, it has also helped me to understand much more about computer programming and the creation of virtual realities. This has been an extremely interesting course to partake in. In the future, if I need to create a website, I am sure that this prior knowledge will come in handy. Overall, this task has been a veritable treasure trove of new information that I am guaranteed to use in the future.
Conclusion:
These activities have been greatly interesting, and I will try to engage in them more wholly the next time an opportunity like this arrives.
Groups: