Aluminium Blog
My partner and I decided to investigate the atom ‘Aluminium’ and proceeded to research about. Through our research, we found that aluminium is a chemical element in the boron group with the symbol Al and atomic number of 13. It is a silvery-white, light-weight metal that is soft and malleable (Royal Society of Chemistry). Aluminium is the third most plentiful element in the earth’s crust, comprising 8% of the planet’s soil and rocks (Lenntech). In nature, aluminium is found only in chemical compounds with other elements such as sulphur, silicon and oxygen, however pure metallic aluminium can be economically produced from aluminium oxide ore or extracted from bauxite by electrolysis (Australian Government – Geoscience Australia). There are several uses of this metal including packaging materials such as foil and cans as well as the construction of transport i.e. automobiles, aircraft fuselages etc. (HowItsMade).
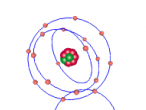
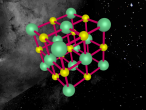
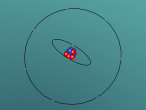
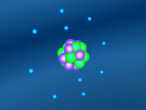
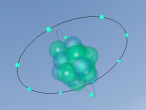
Aluminium is a pure substance, which means that it is composed only of one type of particle; entirely aluminium (Chemistry Index). The atom consists of 13 electrons which are arranged on three orbitals around the nucleus. This is because the first shell can only hold 2 electrons and the second shell can hold only 8 electrons; hence the third shell (Compare Metals). Through research, I found that the arrangement of these electrons around the shells is not necessarily determined; whilst some diagrams show that the electrons are evenly spaced around the shell, others show that the atoms may be clustered in one area of the shell or unevenly spaced. Our programmed model of the atom resembles the evenly spaced structure.
Aluminium is a metal in the Boron family (group III A). It is low in density, hence light in weight, with an atomic mass of 28.98. It is not radioactive, however it has ideal thermal and electric conductivity. Aluminium is very strong and malleable which enables it to be adapted into different shapes and used as a major construction material (Aluminium Design). An interesting property of this element is that it combines slowly wit oxygen to form aluminium oxide. This forms a very thin, whitish coating on the metal which prevent it from reacting further and corroding (rusting). Aluminium reacts with many hot acids as well as alkalis. It also reacts quickly to hot water and when in powdered form can catch fire quickly when exposed to a flame.
Something that I would like to investigate further is the molecules that atoms make up and how its chemical properties contribute to its ability to bond with other atoms.
While the programming experience was enjoyable and educational, there were a few difficulties that were encountered. To begin with, my partner and I had initially chosen the atom Copper to investigate and remodel, however we were required to change. This is because copper has four shells and would be difficult and time consuming to create. Therefore, we chose aluminium which consists only of three shells and would be easier to program. I still found it difficult to operate the program as I personally do not enjoy coding and find it difficult to remember the formulas. I was finally able to accomplish a result with the assistance of my partner and Dr Yeh.
Reference List
http://www.lenntech.com/periodic/elements/al.htm
http://www.australianminesatlas.gov.au/education/fact_sheets/aluminium.html
http://voyager.dvc.edu/~lborowski/chem108index/introductory/Matter_Transformations.htm
http://www.comparisonofmetals.com/About.aspx
http://www.aluminiumdesign.net/why-aluminium/properties-of-aluminium/