Ammonia by Raymond Liu Version 2 (Final)

Ammonia, also known as NH3, is a basic chemical compound that is a molecule made up of three hydrogen atoms and one nitrogen atom. It has a melting point of -77°C and a boiling point of -33°C. At room temperature it is a transparent gas and is characterised by its extremely strong smell. Ammonia plays an important role in the nutritional value fertilisers and foods as it is the precursor. Ammonia is also used to create the synthesis of various pharmaceuticals and is therefore used in the creation of many cleaning products.
Nitrogen is one of the atoms that is found in ammonia. Nitrogen has 7 electrons, 7 protons and two stable isotopes: one with 7 neutrons and one with 8 neutrons. The relative atomic mass of nitrogen is 14.0067 and an average density of 1.251 g/L. Nitrogen has a melting point of −210.00°C and a boiling point of −195.795°C making it a gas at room temperature. It can be found naturally in the air we breathe and is transparent and odourless and was found to be a separable from air by Scottish physician Daniel Rutherford in 1772.
Hydrogen makes up the remainder of ammonia (excluding nitrogen). Hydrogen is a very basic atom consisting of 1 electron and 1 proton. Hydrogen is the only element on the periodic table that has different names for it's different isotopes. This applies for the first three isotopes of hydrogen: 'Protium' refers to the isotope of hydrogen with no neutrons, deuterium refers to the isotope with 1 neutron and tritium refers to the isotope of hydrogen with two neutrons. Protium, deuterium and tritium are the only naturally occurring isotopes of hydrogen. Protium and deuterium are both stable but tritium is radioactive with a half-life of 12.32 years. Tritium undergoes β− decay or beta decay until it becomes stable and transforms into helium 3. Hydrogen has a melting point of −259.16 °C and a boiling point of −252.879 °C. At room temperature it is a colourless odourless gas. It has a relative atomic weight of 1.00794 u with a density of 0.08988 g/L.
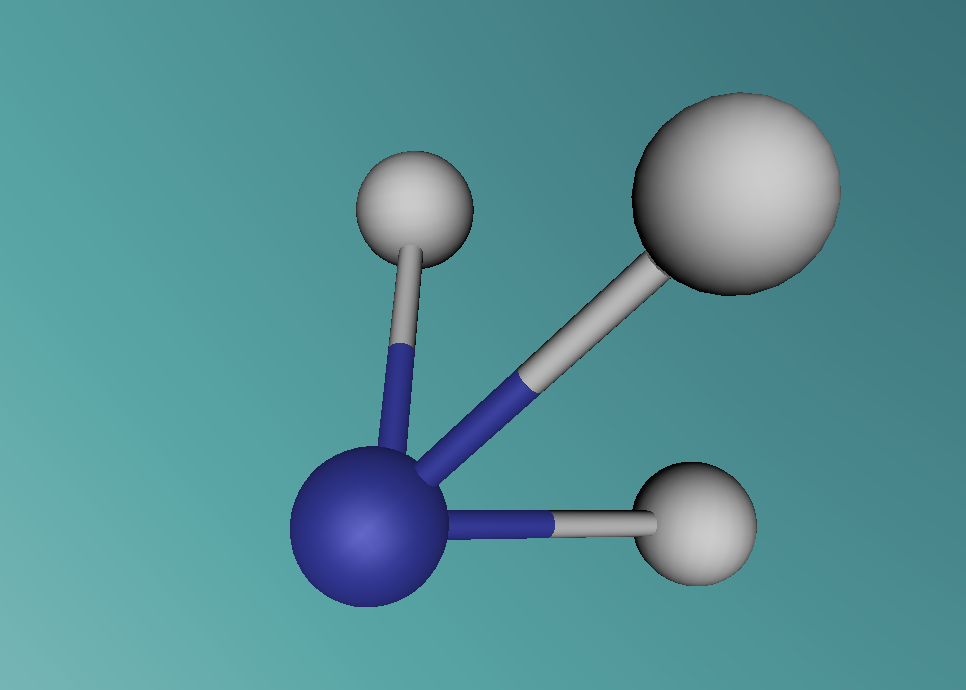
Ammonia, as mentioned before, is made up of three hydrogen atoms and one nitrogen atoms with hydrogen bonds forming between the nitrogen and the hydrogens but not between the hydrogens (see program linked). Through VSEPR (valence shell electron pair repulsion) theorising and experimental testing it has been shown that these bonds form at an angle of 106.7 degrees. The nitrogen in the centre of ammonia has 5 electrons plus one electron electron from each hydrogen given the molecule a total of 8 electrons. These 8 electrons then split into 4 electron pairs that are arranged in a tetrahedral format. Because it can for hydrogen bonds, ammonia can be miscible with water.
Successfully developing a program to map out ammonia was difficult as there were many complications surrounding the rotation of the electron around the different atoms. The biggest problem encountered was making the different shells of electrons around the nitrogen follow different paths instead of being stuck together. This issue was not overcome by the end of the day and still remains unsolved. Questions that could be investigated further could include what kind of chemical reactions involve ammonia and other uses for ammonia
Groups:
























Comments
Program link