Ammonia by Raymond Liu

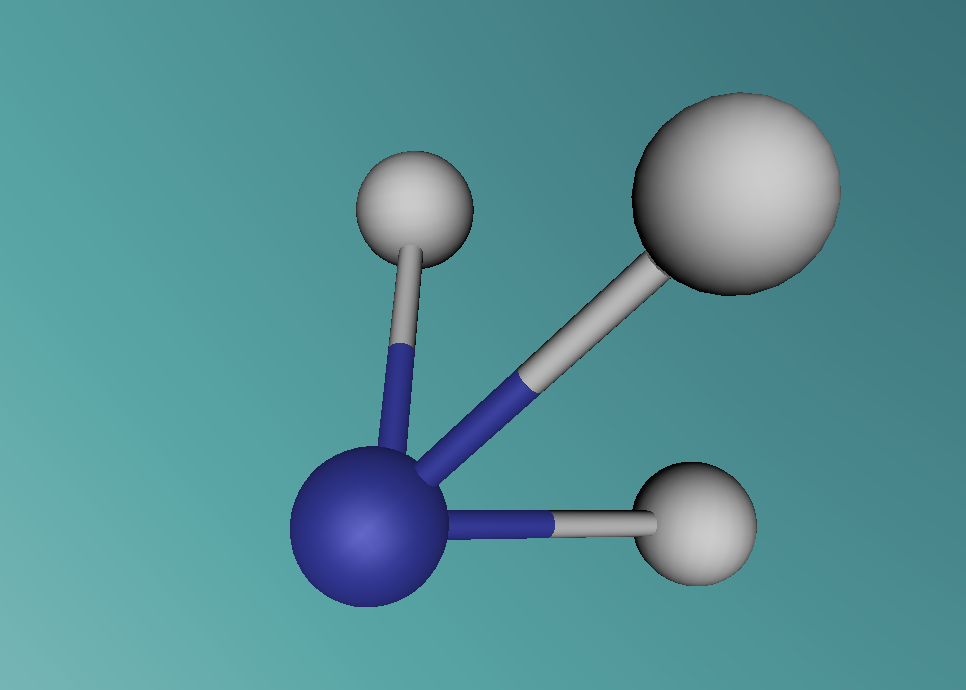
Ammonia, also known as NH3, is a basic chemical compound made up of three hydrogen atoms and one nitrogen atom. It has a melting point of -77°C and a boiling point of -33°C. At room temperature it is a transparent gas that as a extremely strong smell. Ammonia plays an important role in the nutritional value fertilisers and foods as it is the precursor. Ammonia is also used to create the synthesis of various pharmaceuticals and is therefore used in the creation of many cleaning products.
Groups:
























Comments
Draft
This is my first temporary draft
Nice screenshots
Hello rliu11,
You have made nice 3D models. It would be better if you can add the 3D models on your blog, instead of pictures. To do so, you can reference the Handbook from page 24. The handbook is available from the group home page (https://vrmath2.net/BSHS-Y9-Chem) or a direct link here https://vrmath2.net/sites/default/files/Aspire%20Science%20Workshop%20Handbook.pdf.
Also I am wondering why a nitrogen needs three hygrogens to bond together. Is there anything to do with the number of electrons? Can NH2 exist naturally?
Andy