Design
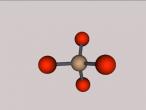
Sulphuric Acid
Sulphuric Acid
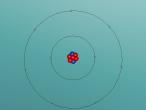
Sulfur atom by Grace Dowdle
In this blog post, I will be informing readers about the composition, structure and characteristics of a chosen atom, sulfur, and I will display the 3D model that myself and my partner created.
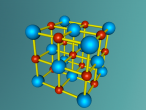
NaCl Lattice Blog Entry FINAL (Ryan Gray ASC091C)
By Ryan Gray ASC091C
This model is of a salt crystal lattice, with the chemical formula NaCl. Its different properties allow it to have many uses in society, including as a preservative, flavouring and currency. Salt demonstrates ionic bonding and its structure makes it interesting to model, because the crystal lattice can continue on forever and doesn't have a specific number of atoms like water does, with each water molecule consisting of one oxygen and two hydrogen atoms. For the purposes of this project, I limited my model to a 3x3x3 cube so it could be easily seen. The distance between the atoms was also expanded to allow the bonds to be more clearly viewed.
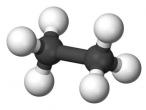
Ethane Molecule
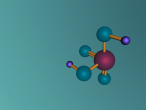
Ethane is a compound that is a byproduct of petroleum processing, and can then be processed to create plastics and ethanol. It is a versatile chemical, and it is also found on other planets, not just Earth. This discovery has scientists interested in Ethane. Ethane’s chemical formula is C2H6 as shown in the diagram below. This blog will discuss the properties, composition, characteristics, and structure of ethane, as well as the uses of ethane, and compounds derived from ethane, as ethane is mainly converted into other molecules.



















 I
I