Quartz Molecule
All matter in the world is compounded from basic units of measurements called atoms. Atoms are made up of protons and neutrons, making the nucleus in the centre and electrons, which are much lighter in rings around it. Elements are substances made from only one type of atom, although when different atoms combine, they form compounds, or molecules. In a compound the atoms are being held together with chemical bonds. In this blog, I will be talking about the structure and characteristics of the molecule quartz.
Quartz Molecule -
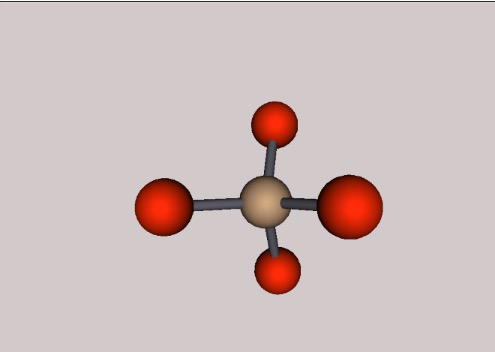
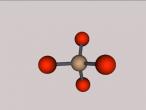
Quartz is a molecule comprised of four oxygen atoms surrounding a silicon atom, forming a tetrahedron. The chemical equation for a singe quartz molecule is SiO4, although in a group of tetrahedra, each oxygen atom is actually shared between two different tetrahedra, making the overall chemical composition SiO2. Oxygen has an atomic number of 8 and an atomic mass of 16 while Silicon has an atomic number of 14, and an atomic mass of 28. This means the centre of the molecule is much heavier than the The individual oxygen atoms surrounding the silicon atom. At room temperature, quartz is a solid and is insoluble, meaning it cannot be dissolved. Even at high temperatures, quartz is a very strong substance, this is due to its strong bonds and macromolecular structure. The chemical bonds in quartz are also covalent, meaning that electrons are shared between the atoms, making the molecule ultimately stronger. In quartz, two electrons are shared between these covalent bonds. The boiling point for quartz is at 1670 degrees Celsius.
There are over 15 different types of quartz as it is so abundant and is found almost anywhere, these all range in colours, luster and transparency. While Pure quartz is colourless and transparent, smokey quartz is grey and opaque. Quartz is the second most abundant mineral in the world, it is highly durable and can form in any temperature. It is widely used as it is heat resistant and very durable, having a 7 on MOHs scale. This means it can be used for electrical products, for making glass or being used as a gemstone. Quartz sand is also used as a filler for paint, rubber and putty, while it can also be found in netball courts, baseball fields and golf courses.
Using the technology on the programming website, VRMaths 2.0. My partner and I were able to create models of different atoms and molecules. The quartz molecule did not seem too difficult at first as it only has four atoms, although creating the right angles in a tetrahedron created quite some difficulty, we were met with assistance and had many trial and error processes in the workshop. The language involved in making the model was also tricky as my partner and I had never done any programming of any kind before. Overall the VRMaths 2.0 experience was interesting and I learnt many new concepts and techniques of programming and designing atoms and molecules.
Overall, I leant much more about atoms and molecules, and how to use the program VRMaths 2.0. I learnt much more about the substance Quartz and about its chemical properties and composition,
further Questions to investigate-
What is the process of creating quartz into glass?
is quartz a valuable substance?
are there any substances similar to quartz? If so what are they?
Websites that were used in this blog and that will provide further information-
http://www.quartzpage.de/gen_chem.html
http://geology.com/minerals/quartz.shtml
http://www.mt-berlin.com/frames_cryst/descriptions/quartz%20.html
https://en.m.wikipedia.org/wiki/Quartz
http://www.quartzpage.de/gen_struct.html
- Lara's blog
- Login or register to post comments
- 14762 reads