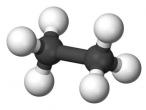
Ethane Molecule
Ethane is a compound that is a byproduct of petroleum processing, and can then be processed to create plastics and ethanol. It is a versatile chemical, and it is also found on other planets, not just Earth. This discovery has scientists interested in Ethane. Ethane’s chemical formula is C2H6 as shown in the diagram below. This blog will discuss the properties, composition, characteristics, and structure of ethane, as well as the uses of ethane, and compounds derived from ethane, as ethane is mainly converted into other molecules.
The composition of Ethane is C2H6, as mentioned above. Because of this chemical composition, there are six hydrogen-carbon covalent bonds, and there is one carbon-carbon covalent bonds. The reason why these are covalent bonds is because the electrons are shared, rather than being given. So, because each carbon atom is surrounded by 4 atoms, it has an additional 4 shared electrons, meaning that there is 8 electrons in the outer shell. Also, because each hydrogen atom is bonded to a carbon atom, the hydrogen atoms each gain one shared electron so that there are two electrons in the hydrogen atoms’ shell.
As mentioned at the start, Ethane is a compound that is a by-product of processing petroleum. It is then converted to ethene(previously called ethylene), a simpler compound that is used to create polythene, and ethanol. The process used to convert ethane to ethene is called steam cracking. This process involves heating the ethane to a temperature above 800 degrees Celcius, and diluting the ethane with steam. The mixture flows through the cracking plant, resulting in ethene. The mixture is then cooled down, to avoid further reactions. In the process of steam cracking, the single bond between carbon atoms is replaced by a double bond.
In order for ethene to be processed into polythene, it requires polymerisation. For polythene, this involves a very high pressure, a temperature above 200 degrees Celcius, and the initiator. In this case, the initiator is a small amount of oxygen, which acts as an impurity. This causes anywhere between 2000 and 20000 ethene atoms to join together, creating polythene. Polythene is commonly used for plastic bags, and for thin, flexible plastic sheets.
Ethene can also be converted into ethanol by mixing it with steam, as shown in the chemical equation below. Ethanol is then used in some types petrol, and in Australia, it varies from E10(up to 10% ethanol) to E85(up to 85% ethanol). Ethanol can also be used in methylated spirits, a cleaning product, and in perfumes. Ethanol is extremely flammable.
Ethane is an odourless, colourless gas at room temperature, but it is extremely flammable. It melts at a temperature of -181.76 degrees Celcius, and boils at a temperature of -88.6 degrees Celcius. Ethane was first discovered by Michael Faraday, in 1834. However, it isn't only found on Earth. Ethane has been found Titan, Saturn’s largest moon, in a hydrocarbon liquid lake. This is because the temperature on Titan is around -179 degrees Celcius, just hot enough for ethane to remain as a solid. It is thought that there may have been ethane rain, to contribute to the lake.
While I was creating this model, I encountered a few small problems. I realised, after creating half the molecule, that the scale wasn't correct, and so I tried to change it, but in the end I thought that it would be more efficient to simply restart. Also, I had problems rotating it, so I had to look at an example atom to find out how to rotate the ethane molecule. Other than that, I created the molecule without any complications. I thoroughly enjoyed the workshop at QUT, as I received the opportunity to learn a new coding language(logo).
I wish to find out more about how ethane is prepared, and also the differences between ethane and other alkanes(hydrocarbons that are by-products of petroleum). The links below are for some websites that contain helpful information about ethane.
http://www.innovateus.net/science/what-ethane
http://www.rsc.org/chemistryworld/2014/10/ethane-podcast
http://www.gcsescience.com/o10.htm
Groups:























Comments
Feedback
Good analysis. in-text? Your 3D model is very well-crafted