Introduction
Methane. (Science: chemistry) A light, colourless, hydrocarbon which is toxic by both inhalation and skin exposure. Also infamously known for being the main substance found in flatulence (the bodily function is actually made up of 59% nitrogen and 21% hydrogen, just saying.) Nevertheless, this blog will explore the structure, composition and characteristics of Methane. Methane contains carbon and hydrogen in the ratio of 1:4 respectively. Since methane is both a powerful greenhouse gas and the simplest and most effective hydrocarbon. It is the main gas found in natural gas piped for use in homes, used to generate electricity and is even used as rocket fuel.
Composition
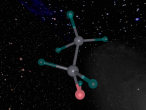
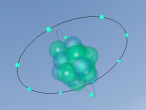
Methane can be demonstrated by the chemical formula CH4, meaning that it consists of a single carbon atom surrounded by four hydrogen atoms. This indicates that it is a molecular compound as it contains five atoms and two types of elements. Carbon (C) is the 6th element in the periodic table and is nonmetallic and tetravalent. This element has 4 protons, 4 neutrons and 4 electrons. Carbon is capable to have four covalent chemical bonds (tetravalent) as it's valence shell (2nd shell) has 4 of 8 electrons. In the case of methane, a carbon atom bonds covalently with four hydrogen atoms. Each of the four hydrogen atoms share their electron with the carbon atom. Furthermore, the carbon atom shares one electron with each hydrogen atom. This fills the valence shell of all five atoms making them stable. Hydrogen (H) is the first element in the periodic table of elements having 1 proton, 0 neutrons and 1 electron. As it only has one shell, it requires an additional electron to fill it's outermost shell. In the molecule Methane, the additional electron is obtained from carbon when the covalent bond is formed.
Structure
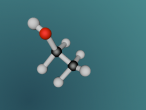
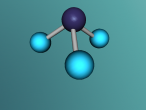
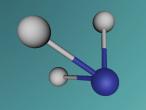
A molecule of methane is arranged in a tetrahedral formation, where a hydrogen atom is placed at each vertex (equal distances apart). At the exact centre of the four hydrogen atoms is a carbon atom, as it needs to provide for the other atoms. Each hydrogen atom is attached to the carbon atom by a single bond. Through the covalent bonding, each atom receives one extra electron for every bond it has (in this case) and the valence shells are completed. The centre of a methane molecule is much denser than the rest of the molecule as it has a mass of approximately 12 opposed the the mass of four hydrogen atoms which is approximately 4. Methane is the simplest hydrocarbon/alkane as it only contains single bonds and has hydrogen atoms everywhere that is possible. Hydrocarbons can be displayed by the general formula of CnH2n+2, which correlates with methane's formula CH4 or C1H1x2+2. Hydrocarbons are also symmetrical in shape and are highly combustible.
Characteristics
Methane is colourless, odourless and flammable. Methane is the simplest member of the paraffin series of hydrocarbons. Its chemical formula is CH4. It is lighter than air, having a specific gravity of 0.554. It is only slightly soluble in water and burns in air, forming carbon dioxide and water vapour; the flame is slightly pale and luminous and very hot. The boiling point of methane is -162.0° C and the melting point is -182.5° C. Methane in general is very stable, but mixtures of methane and air, with the methane concentration between 5 and 14 percent, are explosive. Explosions of such mixtures have been frequent in coal mines and have been the cause of many mine disasters. If you are exposed to air with a high concentration of methane, it is capable of displacing the oxygen in your lungs and cause oxygen deprivation as a result of being a simple asphyxiant.
For more information about atoms, molecules and methane, follow the links provided below:
http://www.c-f-c.com/specgas_products/methane.htm
Bioenergy and renewable power methane in integrated 100% renewable energy systems - by Michael Sterner
http://hyperphysics.phy-astr.gsu.edu/hbase/organic/alkane.html
Interesting ideas / difficulties in maths & programming learnt
The 'logo' programming language was interesting as it was focused on objects and models. As a result, it took me a while to understand that the avatar was a pointer or marker for placing objects. A difficulty in mathematics was finding the angles of the shape. This is because there were four atoms equally spaced coming out of a central atom, although this seemed simple to begin with, we realised that the shape was not just a few two dimensional shapes, and had to attack as a 3D shape in order to create a regular tetrahedral shaped molecule. Using trigonometry, we identified the angle between any two bonds to be 109.5 degrees. Having said that, I learnt that you should always check your work for errors whilst programming as it can be extremely tedious and time consuming to identify an error from a large manuscript.