Methane
The Methane Molecule - E-Yong Lee
Introduction
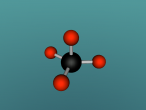
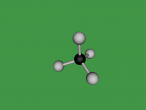
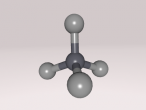
Methane. (Science: chemistry) A light, colourless, hydrocarbon which is toxic by both inhalation and skin exposure. Also infamously known for being the main substance found in flatulence (the bodily function is actually made up of 59% nitrogen and 21% hydrogen, just saying.) Nevertheless, this blog will explore the structure, composition and characteristics of Methane. Methane contains carbon and hydrogen in the ratio of 1:4 respectively. Since methane is both a powerful greenhouse gas and the simplest and most effective hydrocarbon. It is the main gas found in natural gas piped for use in homes, used to generate electricity and is even used as rocket fuel.

FINAL Methane Blog by Anna Lei (ASC092A)
The compound methane has the chemical formula of CH4. Methane is the simplest hydrocarbon of the paraffin series and a potent greenhouse gas, as well as being a large component of natural gas. The major human-associated source of methane is the production/combustion of coal (Britannica, 2016). In nature, methane is the product of anaerobic bacterial decomposition and certain human/animal activities (7% methane in flatulence). It can be found in wetlands, termites, landfills, volcanoes and oceans. The abundance of methane makes it a widely used fuel for heat and light (energy) production (ARM, 2016). In this blog, the details of methane will be uncovered.
- 2 comments
- Read more
- 4189 reads