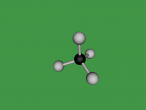
Methane Molecule
Atoms exist all around us, whether it be in living things, or in inanimate objects. They are the basic building blocks of life. However, an atom comprises of a proton, neutron and electron. Protons are positively charged sub-atomic particles, electrons are negatively charged and neutrons have no charge. Atoms can also combine with compatible atoms, to form molecules. Molecules can either just comprise of an element, one type of atom, or a compound, multiple types of atoms. A common molecule found in the air is methane. Methane is an green house gas that is the seventh most prevalent gas in the ozone layer. Methane is used for heat and light, utilising its flammability. Methane comprises of Carbon and Hydrogen. But how do we actually identify methane in the air? Well there are two ways; the composition and structure, and the characteristics.
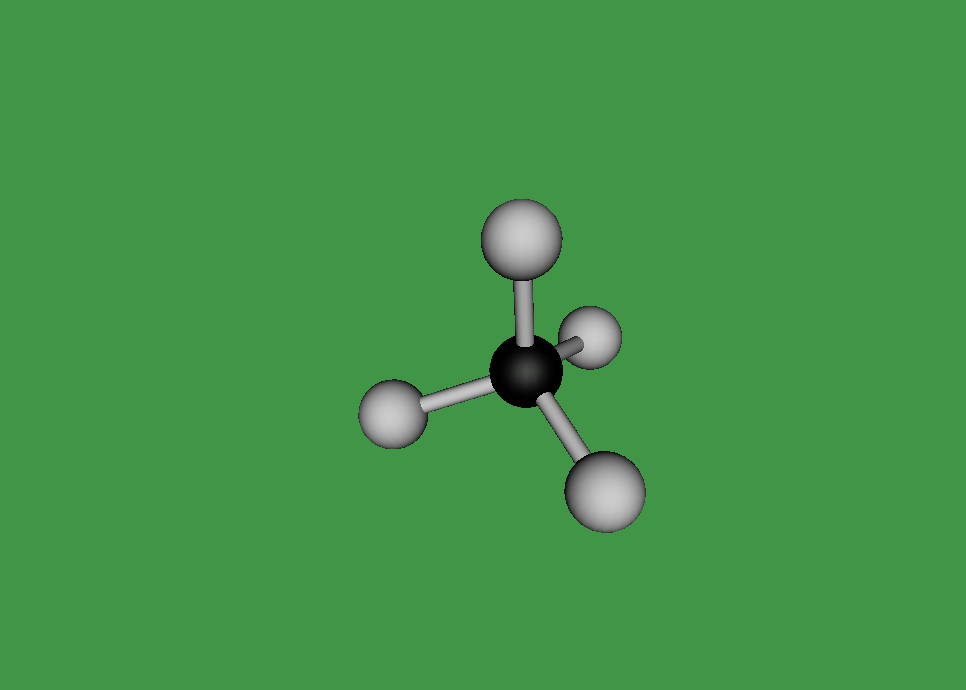
As mentioned earlier, a methane molecule is a compound made up of one carbon atom and four hydrogen atoms and its chemical formula is CH4. The four hydrogen atoms are equivalently connected to the carbon atom, through a C-H bond. A C-H bond is where the outermost electron of a hydrogen atom is shared with the carbon atom to form a single covalent bond between the carbon atom and the hydrogen atom.
The characteristics of methane help identify it from other gases. Methane is a flammable greenhouse gas that burns with a pale, luminescent flames and is odourless and colourless. In terms of mass and density, it's lighter than air, averaging a molar mass and density of 16.043 and 0.668kg/m3. Methane melts at -182 degrees Celsius and has a boiling point at -161 degrees Celsius. Methane also has an auto combustive temperature of over 500 degrees Celsius. While undergoing complete combustion, Methane undergoes an exothermic reaction, which occurs when a molecule of methane and a molecule of oxygen react with each other, which results in the product of water, carbon dioxide and significant amounts of energy. This can be written as CH4 + 2 O2 = CO2 + 2 H2O. While combustion, methane effectively serves its purpose as a fuel, as it exerts a significant amount of energy, during its exothermic reaction, so much so, that in 2009, Germany produced enough biogas to power 3.5 million homes. This brings me to another point.
Methane, stored under the ground, is a non-renewable energy source, which makes it an if deceive source of energy. However, making an everlasting methane farm isn't as inconvenient or tricky as expected, aside from the environmental impacts it may lead to, as a natural producer of over 300L of methane a day.......are cows. Considering that there are over 29 million cattle in Australia, these cows produce 8,700 million L of methane a day. However, all this methane isn't being used and is just being wasted. If this energy was somehow harnessed to power Australian homes and was refined to have a lesser impact on the environment, over 2/3 of Australian homes would be powered for a year with just methane produced by cows. Apart from the impacts on the environment, theoretically, methane could be an effective renewable energy source.
Mostly, programming on VRMath was simple and effective as it required simple commands. However, there were some aspects of the program that were confusing and/or difficult to use. For example, wanting to travel in a certain direction at a certain degree would prove to be difficult, as first, the turtle needs to face a direction, hence needing to turn to face the direction, figuring out the angles that were needed, travelling in that direction and turning again before placing the cylinder where required. This I felt was a bit too complicated, and an option of just turning it manually with a rotating icon would have been helpful. Programming aside, the atom was a bit complex in its design as finding out each angle required time and effort. These were all minor problems however, and VRMath in general was easy and simplistic.
Here are some further links on the topic:
https://www3.epa.gov/climatechange/ghgemissions/gases/ch4.html
https://pubchem.ncbi.nlm.nih.gov/compound/methane#section=Top
Groups: