Sodium Chloride Molecule

Atoms are the basic building blocks of matter, fundamental and key to the formation of everything that exists around us. They make up the air, our bodies and even the very screen that you are staring at right now. Each atom has its own unique atomic structure along with its different characteristics.
These atoms join together into groups to form molecules of either elements or compounds. Elements can only consist of the same type of atoms that cannot be broken down into simpler substances, whereas compounds are made of two or more elements that are chemically bound together. An example of an important molecule in everyday life is Sodium Chloride or commonly known as salt.
What is Sodium Chloride?
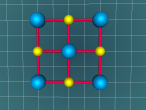
Sodium Chloride (Salt) is a clear-white mineral in the form of cubic crystals, composed of two elements; sodium and chlorine. With the chemical formula of NaCl, salt crystals form lattices that repeat the specific arrangement of the two elements. The salt lattice model below demonstrates the ionic bonds and structure of the molecule. As the lattice is infinite, I have limited the model to a 3 x 3 x 3 cube to allow an easier understanding. Additionally, in real life salt is tightly bound together through the ionic bonds, however in this model the distance between the bonds and size of bonds have been increased to allow a clearer view of the structure.
In the model above, the red balls represent the Sodium atoms and the green balls represent the Chlorine balls. The yellow cylinders represent the bonds between the atoms.
It is commonly known that sodium chloride is comprised of one sodium and one chlorine atom. But why do they bond?
This is because the sodium and chlorine atoms bond together through a particular type of bond called an ionic bond. For an ionic bond to occur, a positively-charged atom, called a cation, needs to attract a negatively charged atom, known as an anion. In this interaction, the sodium atom acts as the cation and the chlorine atom acts as the anion. When this occurs, sodium gives off an electron (a negatively charged subatomic particle) to chlorine. This is because the sodium only has 1 electron in its outer shell, while the chlorine atom is missing one electron to complete its outer shell. Thus when the bonding occurs, it makes the sodium slightly positive (decrease in electrons) and chlorine slightly negative (increase in electrons) making the molecule more stable.
The lattice of sodium chloride is created when multiple NaCl molecules join together to form a cube structure. Each of the chlorine atoms are surrounded by 2 sodium atoms in each of the X, Y and Z planes and vice versa with sodium surrounded by chlorine atoms. the attraction of the positive and negative ions (atoms that have a different number of electrons to protons) causes the molecule to be extremely stable, as the chlorine atoms touch as many sodium atoms as possible without touching any other chlorine atoms.
Characteristics and Uses
Sodium chloride has some interesting characteristics and properties that are reflected by it structure. The strong bonds between the atoms mean that the melting and boiling points are quite high, 800.8° C and 1,465°C respectively. The molecule has a molar mass of 58.44 g mol^-1 and a density of 2.16 g/cm³. It also has the ability to conduct electricity due to its positive and negative ions. Odourless and recognisable through a distinct taste, Sodium Chloride is also soluble in water.
Salt is a used in many aspects of everyday life, from seasoning the mashed potato on your dinner plate to large industries that use salt to create chemicals. Other uses of salt include water conditioning - to balance out other elements, and the manufacturing of materials such as paper as chlorine is used as a bleach. Salt can be used as a road de-icer, as it reduces the melting point of ice and snow, allowing it to easily melt away. It is also used as a preservative for foods as it draws moisture away and kills bacteria.
Despite researching, some questions still remain unanswered in my head. I would like to find out why the chlorine atoms cant touch other chlorine atoms or vice versa, and if there are any differences bewteeen seasalt and mining salt. I would also like to fin out if there is a limit to the latice or if you could have an odd amount of rows for the lattice model.
Overall, the process of construction went quite smoothly, however I did encounter a few difficulties along the way. One of these was finding a way to write all the coding in a succinct and efficient way as the coding was fairly long. This was solved by finding a method to define a specific component of the design so that I only had to write the labelled component, and using the repeat command when necessary. Another problem that I occasionally ran into was locating and moving the reference turtle to a specific location, but this was solved by using the cardinal points and commands such as tilt. I found the concept of using a turtle or moving object marker to display and place objects as a convenient and effective method.
The VRMath Workshop at QUT was a beneficial learning experience that taught us simple coding using the basic logo code and allowed us to assemble a model in a 3D perspective.
Further information can be obtained from the useful sitesprovided below, that assisted me in my research and understanding.
http://study.com/academy/lesson/what-is-sodium-chloride-definition-structure-formula.html
https://www.seasalt.com/salt-101/what-is-salt/
https://www.britannica.com/science/salt
http://www.maldonsalt.co.uk/About-Salt-The-many-uses-of-Salt.html
By Timothy Li - ASC092C
Logo Code
x3D model
LOGO coding: