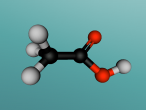
Acetic acid molecule
There are millions of things that exist on Earth, all of which are formed by atoms which are basic building blocks of matter.Every atom contains its unique atomic composition as well as chemical and physical properties. They combine to form molecules and crystal lattices in the form of an element or compound. The different combination of microscopic atoms create different substances.
An element comprises multiple atoms of the same kind and cannot be broken down to a simpler type of matter by chemical or physical means, whereas a compound consists of two or more types of atom and is able to be simplified to elements by chemical means. The combination of different compounds or elements creates another new substance known as a mixture, which can be divided back to its simplest form by physical and chemical means. Molecules can range in size, from the single bond of two atoms to polymers and DNA. An example of a molecule that makes up a commonly used substance known as vinegar is acetic acid.
Acetic acid- what is it?
During the process of researching and building the 3D model, I encountered a few difficulties relating to the measurements of angles and finding the accurate ratio of the measurements between each bond. However, I later on solved these problems by calculating the approximate angle and ratio and consolidating it with my project partner. The biggest problem I had to face when building the model was learning how to program the 'turtle' to position the object in the accurate location. This was the hardest part as I was unfamiliar with the codes and their functions. However, it became a lot easier later on as many of the codes were repeated to produce a similar product.
- mfang4's blog
- Login or register to post comments
- 6061 reads