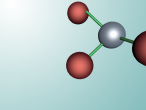
Aluminium Chloride
A molecule is the smallest particle in a compound which is comprised of the same chemical properties of said compound. Molecules are composed of various atoms which are chemically bonded together and thus, alternate in both complexity and size.
Aluminium chloride (AlCl3) is an inorganic compound formed through the covalent bonding of Aluminium (Al) and Chlorine (Cl), which is both produced and employed during the manufacturing of aluminium metal. During the production process, the aluminium undergoes an exothermic reaction, reacting with chlorine under high temperatures, as shown by the formula 2 Al + 3 Cl2 → 2 AlCl3. However, aluminium chloride is also commonly used in the chemical and cosmetic industry, the latter of which uses it to produce deodorant. This is because aluminium chloride is a strong aluminium salt and is known to inhibit sweat. It often appears as a yellow or white power and has a strong odour. Aluminium Chloride can be found in three different states: solid, liquid or gas. When aluminium chloride changes state from a solid to a liquid/gas, it changes structure from a covalent structure to a ionic structure. Solid aluminium chloride is structured in a six-coordinate layer cubic lattice. Once melted, it can produce the dimer Al2Cl6, which is able to vaporise and restructures to a triagonal planar form. Aluminium chloride is highly deliquescent, meaning that it has a tendency to change into liquid form. It has a melting point of 194 degrees Celsius and a boiling point of 180 degrees Celsius. Aluminium Chloride, whilst a strong antiperspirant, is a poor conductor of electricity and is soluble in only few solvents.
Aluminium Chloride is composed of three Chlorine atoms and one Aluminium atom and has a molecular weight of 133.340539 g/mol. As shown on the model above, the molecule is structured in a 'Y' shape, with the aluminium atom in the centre, one chlorine atom branching off the middle of the atom, and two branching off it on the other side. The aluminium atom consists of 13 protons, 14 neutrons and 13 electrons, whilst the chloride atom consists of 17 protons, 18 neutrons and 17 electrons. Due to the equal amount of protons and electrons in all four atoms, aluminium chloride is stable but reacts violently when in contact with water due to the high heat level of hydration. This is because it is self-reactive, which means that is thermally unstable and thus susceptible to a strong exothermic decomposition even without the the presence of oxygen. As oxygen is present in water (H2O), the aluminium chloride undergoes a powerful chemical reaction.
During the construction of the VRmath2 model, many difficulties arose whilst programming. For example, it was difficult to estimate the degree at which an object must turn, or the amount of 'metres' forward the vector must travel. In addition, as the measurement unit used on the programming was unknown, it was difficult to create an accurate model. However, we were taught during the workshop that many atomic and molecular models are inaccurate as we cannot correctly measure the distance between the nucleus and a proton, or an atom and another atom.
After researching aluminium chloride, I would like to investigate why the covalent bonding between the aluminium and the chlorine changes to an ionic bond as well as Furthermore, I would also like to further research why aluminium chloride is used as a deodorant and what effects it has on the human body.
Further Links:
https://www.cs.mcgill.ca/~rwest/wikispeedia/wpcd/wp/a/Aluminium_chloride.htm
http://www.chemicalbook.com/ChemicalProductProperty_EN_CB6247076.htm
https://pubchem.ncbi.nlm.nih.gov/compound/aluminum_chloride#section=MeSH-Synonyms
http://www.masterorganicchemistry.com/2011/07/22/reagent-friday-aluminum-chloride-alcl3/