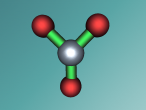
Aluminium Chloride Molecule
Atoms are the smallest particle of an element and are the basic building blocks of all matter. Atoms are composed of a nucleus which contain positively charged particles called protons and no charged particles called neutrons. Surrounding the nucleus are negatively charged electrons arranged in shells. These electrons exist in a cloud around the nucleus. When two electron clouds of different atoms interact, they bind together to form a molecule. An example of a molecular compound (a molecule that contains atleast two or more different elements) is Aluminium Chloride (AlCl2).
Aluminium chloride has a number of characteristics that differentiate it from other molecules. Firstly the melting point of the molecule is 190 degrees celsius and the boiling point is 178 degrees celsius. In its solid state, Aluminium Chloride is a powdery form with varying colours based on contact with moisture and other substances. The most common colour is yellow. Moreover, in it’s solid form it’s density is 2.44 g. The molecule is relatively stable but when water is applied to solid aluminium chloride, a violent reaction occurs with the production of steamy fumes of hydrogen chloride gas. Aluminium Chloride is most commonly used to control excessive sweating in the form of an antiperspirant. When applied to skin, aluminium chloride salt dissolves from the sweat or moisture of the skin surface forming an acidic solution that diffuses into the sweat glands. Then metal ions precipitate and the aluminium chloride hydrolyses in more alkaline sweat, forming a gel plug in the lumen of the sweat gland duct. This prevents pores from releasing sweat.
During my programming process of my molecule I found many interesting aspects of the software, as well as difficulties. Firstly, I found the precision of the programming particularly interesting. If one letter or number was incorrect it caused the whole programme to disfunction. As my program was particularly long, it was especially difficult to find the small errors I had made and alter them. In addition, the idea of using an object, in this case a turtle, as a reference for placing other objects was also interesting. Every object placement, including the angle, shape, colour and direction, was based on the reference object. This made programming considerably easier to manage as a preview of our objects location was given. However with this reference function came a difficulty. No matter which direction the turtle was facing, the North, South, East and West (up, down, forward, backwards, etc.) directions never changed. For example, even if the turtle was upside down, if I commanded the turtle to move upwards it will always move where north was. Thus, I found it difficult at times to place the turtle in the location I wanted my object.