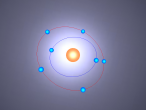
Nitrogen Atom
It is an element that is an essential to all life on Earth, yet in its purest form it can suffocate living organisms. Nitrogen is one of the most important elements on Earth – it can be found in all living systems and makes up around 78% of the Earth’s atmosphere. It is a constituent of protein and nucleic acids. In its gaseous form, nitrogen is colourless, odourless and generally considered inert. In its liquid form nitrogen is also colourless and odourless, and resembles water. Nitrogen was first recognised in 1772 by number of scientists; Carl Wilhelm Scheele, Daniel Rutherford, Henry Cavendish and Joseph Priestley, who all found that air was composed of three different gases, oxygen, carbon dioxide and another gas which they dubbed ‘foul air’ (nitrogen). Nitrogen was first recognised as an element by Antoine Laurent Lavoiser in 1786, and named nitrogen in 1790 by Antoine Laurent Lavoiser. Today, nitrogen is used in food preservation, explosives, and aids medical research.
At the end of this blog post is a model of a nitrogen atom programmed by my partner Emily and I. Note that it is just a model and not completely accurate. There are no protons or neutrons visible in the nucleus, but in a real nitrogen atom there would be 7 protons and 7 neutrons in the nucleus. In the model, either the nucleus is too large, or the electrons are too close. If the model was to be completely accurate the electrons should've been placed a mile away, which clearly is not possible in this situation. In reality scientists have no way of telling exactly where an electron is at any given moment. They can, however, calculate the probability that an electron can be found in given volume of space, and this idea is called the Quantum Theory.
This model contains protons and neutrons in its nucleus - https://www.britannica.com/science/Bohr-atomic-model/images-videos/Bohr-atomic-model-of-a-nitrogen-atom/155372
The element nitrogen is number 7 on the period table, and has an atomic weight of 14.01. It contains 7 protons, 7 neutrons and 7 electrons. Five electrons are in the outer shell and two electrons in are in the inner shell. The nucleus contains 7 protons and 7 neutrons. Because of the few electrons on the outmost shell, it's easy for nitrogen to bond with other elements, creating compounds such as nitrogen oxide and ammonia.
As aforementioned, nitrogen is present in all living organisms – plants, animals and human beings. It is the primary component in amino acids, the building blocks of protein. It is also the basis of neurotransmitters, RNA and DNA. In plants, nitrogen is required in the chlorophyll pigment, which allow plants to convert sunlight into energy.
Nitrogen, in its liquid and solid form, has many uses. At room temperature, it is a noncombustible, nontoxic gas. Thus, it can be used for preserving food and reducing or eliminating oxidation. Nitrogen gas is also used to preserve eggs, blood, sperm and other medical specimens for biological research. One of the most important nitrogen compounds is ammonia, which is created when hydrogen reacts with nitrogen. The colourless ammonia gas can be liquified to create fertiliser. Ammonia can also be used to make refrigerant gas, and used in the manufacture of plastics, textiles, dyes, pesticides and cleaning solutions.
To complete this assignment, we had to be able to program. This was a fairly new topic for me, as I have programmed before but definitely not to this extent of difficulty and never on the website that we had to do our model on. My partner, however, was quite experienced on this topic so she helped me a lot in this area. We also had additional help from the staff at QUT. It was difficult to grasp the whole idea at first, and we had some difficulty, especially when making the rings around the nucleus move, but eventually we got it. The whole workshop was a huge learning experience for me as not only did I learn more about my element but also how to program and make models of atoms and molecules online. Unfortunately, we were not able to make the protons and neutrons visible in the nucleus (the ‘clumped’ nucleus) and this is something my partner and I want to work towards creating in the near future.
I would like to investigate – exactly how accurate can an atomic model be? Is there such thing as a ‘perfect’ atomic model? If so, how was it made? If not, why is it not possible? Perhaps I can answers to these questions in the near future. Meanwhile, I have found some good resources which provide more detailed information about nitrogen.
https://www.britannica.com/science/nitrogen
I also found this article that explains how nitrogen is so essential to Earth, yet at the same time it is destroying the Earth - http://ngm.nationalgeographic.com/2013/05/fertilized-world/charles-text
Thank you for reading by blog post. I hope you have learned something about nitrogen.
Groups: