Engineering
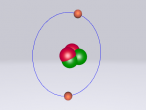
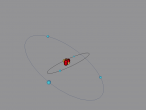
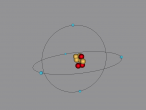
Boron Atom
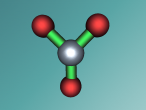
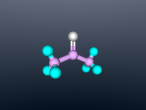
Aluminium Chloride Molecule
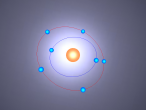
Atoms are the smallest particle of an element and are the basic building blocks of all matter. Atoms are composed of a nucleus which contain positively charged particles called protons and no charged particles called neutrons. Surrounding the nucleus are negatively charged electrons arranged in shells. These electrons exist in a cloud around the nucleus. When two electron clouds of different atoms interact, they bind together to form a molecule. An example of a molecular compound (a molecule that contains atleast two or more different elements) is Aluminium Chloride (AlCl2).
Aluminium Chloride
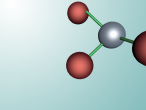
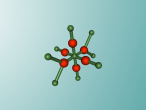
Calcium Chloride
This blog will detail the composition, structure and characteristics of calcium chloride and includes
Boron Atom (By Melody Suen)
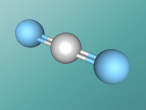
Acetone Blog
My name is Michaela Cheong and I am a student at Brisbane State High School. In our current unit of science, we are learning about chemistry, specifically the structure and characteristics of atoms (building blocks which make up everything) and compounds (consist of two or more atoms which are chemically bonded). In this blog, I will show you a 3D model of a molecule of acetone along with a discussion of it’s molecular structure, uses and properties.