Calcium Chloride
This blog will detail the composition, structure and characteristics of calcium chloride and includes an interactive 3D molecular model of the aforementioned molecule. Calcium chloride is an ionic compound made up of calcium and chlorine and is reflected in the chemical formula CaCl2. It is a inorganic salt and is commonly used as ice and dust control for roads, oil and gas drilling as well as an accelerator for concrete. Other uses for calcium chloride include plant refrigeration and for medicinal purposes such as replenishing calcium levels and an antidote for magnesium poisoning.
Information
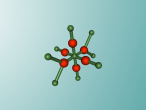
Calcium chloride is formed through the ionic bonds between calcium cations and chloride anions. A single calcium chloride molecule is composed of one calcium ion and two chloride ions. Calcium ions have a charge of +2 whereas chloride ions have a charge of -1, this means that calcium chloride molecules have 0 or neutral charge. Calcium chloride molecules can also form crystals based on these ionic properties. The positive calcium ions orient themselves so that they are close to negative chloride ions from another molecule resulting in the crystalline structure shown in the interactive model.
Issues in Creation of Model
One of the most prevalent problems in the creation of the interactive 3D model was the programming. Although the program wasn't challenging in itself, its extreme repetitiveness meant that over 100 lines of code had to be utilised to create what initially looks like a relatively simple model. This was extremely time consuming and made the work far more laborious than it had to be. Although the obvious solution to this problem would be to utilise the repeat command, it would not work in the case of this program. This is due to the fact that despite being almost exactly the same, each line of repeated code was slightly different. As a result, the repeat command would have to be re-entered continuously therefore negating its desired function.
http://study.com/academy/lesson/calcium-chloride-uses-structure-formula.....
http://www.calciumchloride.co.uk/calcium_chloride_info.html