At the QUT workshop, I learnt how to use VRMath 2.0 to program, create 3D molecules/atoms and how to write a blog. With my partner Hana, we created a 3D model of a boron atom. In this blog post, I will explain the composition, structure and characteristics of boron, as well as the programming used.
Boron (B) is a metalloid element first extracted in 1808 by Louis-Josef Gay-Lussac and Louis-Jacques Thénard in Paris, and independently by Sir Humphry Davy in London. Pure boron appears as a dark amorphous powder and is found in the cell walls of plants, helping with cell wall formation, functioning and strength, so boron deficiency can lead to poor growth and reduced plant development. This element is also important for humans, as its compounds such as boric acid, sodium borate and boric oxide, are used in a variety of products, including eye drops, mild antiseptics, washing powders, tile glazes, bleach and food preservatives. In addition, it is also used in rocket fuel igniters and pyrotechnic flares.
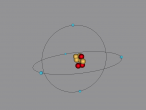
Here is my 3D model of a boron atom:
A boron atom has two shells, and five electrons, with two in the first shell, and three in the second. In the nucleus, there are five protons and six neutrons. Pure boron exists in at least four crystalline allotropes. Closed cages containing 12 boron atoms are arranged in the form of an icosahedron, occurring in the various crystalline forms of elemental boron. Boron consists of a mixture of two stable isotopes: B-10 (19.9%) and B-11 (80.1%).
Pure crystalline boron is black and lustrous and has a Mohs hardness of 9.3 MPa, which is able to scratch some abrasives but too brittle to use in tools. Boron has an atomic mass of 10.81 amu and its density is 2.37 g/ cm³. Some characteristics of boron include a melting point of 2077°C and a boiling point of 4000°C, so it is thermally stable, as it has more resistance to decomposition at high temperatures (high melting and boiling point), therefore, it can be used in rocket fuel igniters. Its heat capacity is 1.026 J/g, so it can be used in pyrotechnic flares, combined with other materials, to create intense light and heat.
If you want to learn more about boron, I have provided some links below for you to read:
http://periodic.lanl.gov/5.shtml
https://www.britannica.com/science/boron-chemical-element
http://www.livescience.com/28674-boron.html
http://www.rsc.org/periodic-table/element/5/boron
http://www.periodictable.com/Elements/005/data.html
I learnt many interesting ideas during this workshop, as I had very little experience with programming, such as how to use mathematics and how to write coding.
However, there were also some difficulties, such as trying to program the nucleus as it was extremely difficult to use mathematics to place each ball (protons and neutrons) so that they were clumped together in the nucleus. It took multiple tries of trial and error before we achieved what we wanted, as we realised that some of the balls merged together if they were put too close together.
Here is my logo program:
boron_final.logo