Acetone Blog
My name is Michaela Cheong and I am a student at Brisbane State High School. In our current unit of science, we are learning about chemistry, specifically the structure and characteristics of atoms (building blocks which make up everything) and compounds (consist of two or more atoms which are chemically bonded). In this blog, I will show you a 3D model of a molecule of acetone along with a discussion of it’s molecular structure, uses and properties.
Structure/Characteristics
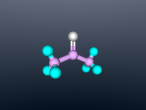
The molecule shown above is a molecule of acetone, which is demonstrated by the chemical formula (CH₃)₂CO and is known by other scientific names such as propanone. The compound is made up of one oxygen molecule (represented by the white), 3 carbon molecules (represented by the purple) and 6 hydrogen molecules (represented by the blue). Acetone is a colourless liquid with a strong smell, flammable properties and that easily evaporates. Because the liquid contains carbon atoms, it is considered an organic compound, containing carbon atoms. It is also classified as a type of ketone, as it contains a carbonyl group made up of the double bonded oxygen and carbon atoms, as well as two hydrocarbon groups on either side.
The compound is a liquid at room temperature, with a boiling point of 56.2°C and a freezing point of -94.8°C. It has a density of 0.7845 g/cu cm, making it less dense than water, but it is miscible with water, meaning that the two liquids make a consistent mixture when combined.
Acetone is considered a polar molecule as the charges between the atoms are unbalanced. Between the oxygen and carbon atom, there is a double bond in which the oxygen atom is left with two unshared electron pairs, leaving the bond with a negative charge. On the contrary, the bonds between the carbon atoms and hydrogen atoms are positive, meaning that the molecule is polar due to the uneven distribution of charges.
Uses
Acetone is a clear, flammable liquid commonly used as an industrial solvent. This means that it is able to dissolve other substances. For this reason, acetone is most commonly used in the cosmetics industry as nail polish remover. Have you ever used an acetone-based nail polish remover? Acetone can also be found in other bath, fragrance, skin-care, hair-care and skin-lightening products. Because of its volatile nature (i.e. it evaporates very quickly), it is used in dermatology to dry the skin of excess fats and oils. Other uses of acetone include: thinning polyester, cleaning tools, dissolving epoxies and superglue pre- or post-hardening, degreasing surfaces, transporting other compounds safely, farming as an agricultural pesticide, .
Further Investigation
Acetone has many uses in pharmeceutics, dermatology, cosmetics and industrial work, however, I would like to further investigate the way that acetone is produced and disposed of in the human body as a metabolic process. It is briefly mentioned on the Wikipedia page, however, I could not find any other information on the subject. It would be interesting to look in to. Below are the links I used:
http://chemistrymolecule.blogspot.com.au/2011/02/moleculechcl3.html
https://www.reference.com/home-garden/uses-acetone-d453ad70cb56bdb6#
https://pubchem.ncbi.nlm.nih.gov/compound/acetone#section=3D-Conformer
http://study.com/academy/lesson/what-is-acetone-structure-uses-formula.html
The Coding Process
Learning how to use the coding program and each function that could be performed was an interesting process. I personally managed to pick up on the coding quickly at first, and it was fun experimenting and using the logo program. Initially, my partner and I had planned to do a different molecule to the acetone molecule above, however, upon coding the model of the molecule, we decided that it was too simple and changed to an acetone molecule.
When it came to coding the molecule, I decided to try and experiment first and make a draft version of the molecule, hoping that it would turn out to be good enough to use post-editing. Upon trying to code the second half of the model, the angles were not the same on both sides and the model was not turning out to be parallel. In light of this, I restarted the model but used mathematics to calculate the angles I would have each cylinder come out of each sphere, making sure that I stuck to the dimensions outlined. Using a mathematical approach to code the molecule was far more effective than experimentation.
Coding of the Logo