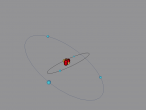
Boron Atom
Boron is the fifth atom in the periodic table, being a metalloid. The rare element, only made as compounds in the nature, has been used commonly for glass and glazes production. While its presence has been known since 1808, the first 99 percent pure boron was made only in 1909. A 3D model of this boron was made using the VRMath2.0, shown below. The Bohr model has been used to represent the element, as it simplifies the characteristics of electron movements. However, the model is not entirely accurate. In the process of programming the atom, many difficulties and challenges were experienced.
Boron is a chemical element, the atomic number 5 and its symbol expressed as B. The origin of its name comes from 'buraq', an Arabic term which is a name for borax. Borax, tincal or sodium tetraborate decahydrate, also is a substance used for glasses and pottery glazes. It is in group 13, period 2 and in the p-block of the periodic table. Pure boron is a dark amorphous powder, which is different to the other members of the group 13, as all the other elements are metals in this group. At room temperature (20ºC), this element is a solid. The melting point is 2077ºC, and the boiling point is 4000ºC. The relative atomic mass is 10.81 and the density is 2.34g/cm^3.
Boron has many uses, both naturally and for humans. It is used in plant cell wall structure. Also, some compounds of boron are studied as a possible treatment for brain tumours, which are unnecessary and abnormal cells within the brain. Boron is used for rocket fuel igniters as well, giving the green colour flare. While compounds of boron were used from before, the first success in isolating boron happened in 1808, by Louis-Josef Gay-Lussac, Louis-Jacques Thénard in Paris and Sir Humphry Davy in London. This boron is not completely pure, but it was classified as the first discovery of boron. Main isotopes of boron are boron-11 and boron-10. In particular, boron-10 is good at absorbing neutrons, so are used to regulate nuclear reactors and have a role in instruments used to detect neutrons.
Boron consists of 6 neutrons, represented by the yellow spheres of the 3D model, 5 protons, red in the model and 5 electrons, the blue orbiting spheres. To represent a boron atom, the Bohr model, created by Niels Bohr in 1913, has been used. This model may lack in accuracy, but simplifies the movement of electrons. In the Bohr model, electrons orbit the nucleus made of neutrons and protons. However, electrons do not orbit the nucleus in well defined circular orbits as used in the Bohr model.
For additional information on boron, visit;
About the Bohr model;
In creating the 3D model, many difficulties and challenged were experienced. Firstly, the particles in the nucleus could not be placed in a circle. This is because the number of protons and neutrons in the nucleus were unnatural, making it hard to create a sphere with 11 particles. Secondly, creating a program to make the electrons orbit the nucleus took time. Some improvements to the model would be to more accurately duplicate an atom. By using a different model, with no well-defined orbits of electrons and a more accurate spacial distribution of particles, the model could be used in educational purposes to see an atom, which is unable to physically see with modern technology.
For the logo of this 3D model; /sites/default/files/user/u377/logo/Final_Boron.logo
For the model itself; /sites/default/files/user/u377/x3d/Boron.x3d
Written by Hana Sato, Year 9, BSHS
Groups: