Chemistry
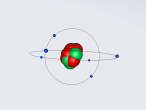
Boron Atom
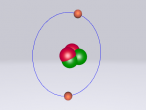
Boron is the fifth atom in the periodic table, being a metalloid. The rare element, only made as compounds in the nature, has been used commonly for glass and glazes production. While its presence has been known since 1808, the first 99 percent pure boron was made only in 1909. A 3D model of this boron was made using the VRMath2.0, shown below. The Bohr model has been used to represent the element, as it simplifies the characteristics of electron movements. However, the model is not entirely accurate. In the process of programming the atom, many difficulties and challenges were experienced.

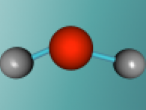
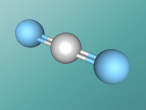
Water Molecule
What if the world had no water?....Water is one of the most crucial substances for the survival of living beings, and takes up approximately 70% of the world's surface. But what is water? Have you ever heard people say the word "H2O" and wondered why water was given that name? Well, "H", "2" and "O" are not just random letters and numbers put together to sound fancy; H2O is the molecular formula for water, representing the individual atoms in the compound. Water is an extremely unique molecule, having an interesting composition, structure and set of characteristics. So, let's flow right into it!
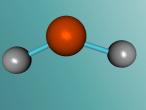
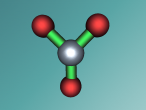
Aluminium Chloride Molecule
Atoms are the smallest particle of an element and are the basic building blocks of all matter. Atoms are composed of a nucleus which contain positively charged particles called protons and no charged particles called neutrons. Surrounding the nucleus are negatively charged electrons arranged in shells. These electrons exist in a cloud around the nucleus. When two electron clouds of different atoms interact, they bind together to form a molecule. An example of a molecular compound (a molecule that contains atleast two or more different elements) is Aluminium Chloride (AlCl2).
Aluminium Chloride consists of three Chloride atoms and one Aluminium atom which is shown in the image below. These atoms are structured in a trigonal planar with the three chlorine atoms surrounding the single aluminium atom with bonds, 120 degrees apart.The structure of the molecule is based upon temperature change. At temperatures around 190 degrees Celsius Aluminium Chloride converts to Al2Cl6. As temperatures increase further, the molecule is broken up into simple AlCl3 molecules. The aluminium and chloride atoms are bonded covalently, sharing electron pairs in the attractive and repulsive forces between the atoms. However this is only the case for the liquid or vapour form of Aluminium Chloride. When it is in solid form the atoms bond ionically, transferring electrons between atoms.
Aluminium atoms (Al) are composed of 13 protons and 14 neutrons in its nucleus, and 13 electrons surrounding the nucleus. The surrounding electrons are arranged in three shells, 2 in the first, 8 in the second and 3 in the third. Aluminium atoms are one of the lightest atoms with an atomic mass of 26.98 amu. Similarly, Chloride atoms have an atomic mass of 35.45 amu. Chloride atoms have 17 protons and 18 neutrons present in its nucleus and 17 electrons surrounding it in 3 shells. The first shell having 2 electrons, the second having 8 electrons and the third having 7 electrons.