What if the world had no water?....Water is one of the most crucial substances for the survival of living beings, and takes up approximately 70% of the world's surface. But what is water? Have you ever heard people say the word "H2O" and wondered why water was given that name? Well, "H", "2" and "O" are not just random letters and numbers put together to sound fancy; H2O is the molecular formula for water, representing the individual atoms in the compound. Water is an extremely unique molecule, having an interesting composition, structure and set of characteristics. So, let's flow right into it!
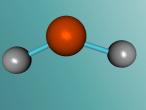
The water molecule is composed of two "H" atoms and one "O" atom: two hydrogen and one oxygen, which are the first and eighth elements on the periodic table. Each hydrogen atom has one electron whilst the oxygen has two in the inner shell and six valence electrons in the outer shell. Due to atoms being most stable when having two or eight electrons, they often combine to form molecules by sharing electrons; this is called covalent bonding. The shared pair of electrons which hold the atoms together are called the "bond pairs" whereas the others are known as "lone pairs". In water's case, oxygen's octet of eight electrons in the outer shell is completed after joining with hydrogen atoms, resulting in four electron pairs: 2 bond pairs and 2 lone pairs. Since the lone pairs are invisible and still exert an influence on the arrangement of the molecule, H2O has a bent, "V-shaped", and rather tetrahedral molecular geometry. In addition, the repulsive forces from the highly-negative lone pairs keep them as far away from each other as possible, compressing the angle between the hydrogens; the bond angle created by the three atoms is approximately 104° rather than the usual 109° of a perfectly tetrahedrally arranged molecule. This gives water a unique molecular structure.
H2O also has many special characteristics that don't frequently occur in other types of molecules. Firstly, H2O exists in all three states: liquid, solid and gas. Due to vibration speeds of particles depending on temperature, at 0°C liquid water freezes as the particles lose energy and become rigidly-packed together. Alternatively, at 100°C, liquid water boils and changes into gas as the particles gain more heat energy and vibrate fast enough for the water molecules to break apart from their hydrogen bonds. Hydrogen bonds are the loose bonds between separate molecules when negatively-charged oxygen atoms in H2O are attracted to the positively-charged hydrogens of other water molecules. The reason oxygen atoms in water act negative and hydrogen atoms act positive is ultimately due to a concept called polarity. Since oxygen has a higher electronegativity (a measure of how much one atom wants to have electrons) than hydrogen, the electrons are shared unequally between the atoms - just like a game of tug-of-war. Electrons are pulled closer to the oxygen atom, causing it to become more negatively-charged while the slightly electron-deficient hydrogens become more positive; thus, because opposites attract, hydrogen bonds form. This ability water has to stick to itself is called cohesion, and its ability to stick to other substances is called adhesion. This concept also explains why unlike other substances, water's solid form is less dense than its liquid form. Ice can float on liquid water because the hydrogen bonds keep the molecules further apart. Furthermore, surface tension due to hydrogen bonding creates a thin resistance that allows some very light insects to walk on water. Unfortunately, as we already know, these bonds are not strong enough to hold up humans.
There is still so much more to explore about this unique molecule with its enigmatic properties. Some interesting topics for further reading include capillary action which allows water to "defy gravity", and heat capacity which explains why the temperature of oceans remain fairly constant or subject to little change from global warming. Another interesting inquiry is investigating whether water itself really does conduct electricity. Feel free to learn more from these links:
http://study.com/academy/lesson/capillary-action-of-water-definition-examples-lesson.html
http://www.bitesizephysics.com/Lessons/heatcapacity.html
http://www.ces.fau.edu/nasa/module-3/why-does-temperature-vary/land-and-water.php
http://www.fallonsolutions.com.au/Handy_Hints/why-water-and-electricity-dont-mix
I had a very memorable experience at QUT as I participated in the VRMath 2.0 Workshop. It was very enjoyable, and allowed me to gain more knowledge in programming and creating virtual molecules and atoms. The workshop really gave me a challenge as I learnt to overcome the difficulties that my partner and I originally faced when using the logo editor. Initially, it was hard to follow the course, and use the program to create our own molecule, as instructions flew at us rapidly; however, as we became more familiar with the basic commands and processes, we were able to successfully complete our task. It was interesting when I realised how mathematics and general patterns were incorporated in the methods used for the logo editor; this simplified the originally-complex logistics involved. For example, at first it was immensely difficult to create a bent bond between the two hydrogen atoms of our water molecule but after finding the right angle with our number skills, this was achievable. I learnt that like designing and building in reality, understanding is developed after time, and mistakes are inevitably part of the process. Thus, there is still room for improvement. Hopefully next time, I'd be able to increase the accuracy and aesthetics of any more models I may attempt to create.