Mathematics
Neon Atom by Jade Upton
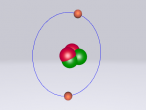
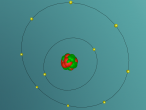
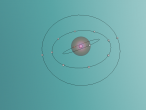
An atom is the smallest particle of a chemical element; so consequently, atoms are the building blocks of matter and the world around us. I have modelled an atom of the chemical element Neon (Ne) through the website VRMath 2.0 using the skills I learnt during the workshop at QUT last Tuesday. The element Neon has an atomic number of 10, and its atom consists of 10 protons, 10 electrons, and in most cases and as depicted in the model, 10 neutrons. My partner Lauren and I decided on modelling this atom because we were intrigued about its properties as we had very recently learned of its existence and its role in neon signs. For this assignment, I created a 3D model of 20Ne using Bohr’s atomic model (below).
NaCl Lattice Blog Entry FINAL (Ryan Gray ASC091C)
By Ryan Gray ASC091C
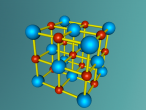
This model is of a salt crystal lattice, with the chemical formula NaCl. Its different properties allow it to have many uses in society, including as a preservative, flavouring and currency. Salt demonstrates ionic bonding and its structure makes it interesting to model, because the crystal lattice can continue on forever and doesn't have a specific number of atoms like water does, with each water molecule consisting of one oxygen and two hydrogen atoms. For the purposes of this project, I limited my model to a 3x3x3 cube so it could be easily seen. The distance between the atoms was also expanded to allow the bonds to be more clearly viewed.
Carbon Dioxide
Carbon dioxide is all around us: the bubbles in soda, dry ice for refrigeration and the gas in some fire extinguishers.
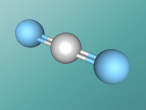
Carbon dioxide has a chemical formula of CO2, was 'discovered' in the 1750's by a Scottish physician Joseph Black and makes up 0.04% of the Earth's atmosphere.
In this blog post I will discuss carbon dioxide; the composition, structure and characteristics as well as its emissions.

HydROgEn cYAnIde
Atoms are the building blocks of all matter that we can touch, affect and be affected by. They each contain varying numbers of protons, electrons and neutrons that make up hundreds of thousands of different elements, molecules and compounds used both by nature and humans. These can range from a simple oxygen molecule (O2) to more complicated compounds such as Methamphetamine, C20H15N. One such molecule is HCN; Hydrogen Cyanide.

2nd DRAFT for MAGNESIUM ATOM
Dear Teachers,
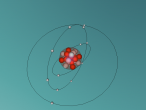
This is our draft of magnesium atom, just to check whther this publishses properly or not (NOT A FINAL). Draft Atomic structure created by Haritha. B and Krupesh. M.
Silicon
Nitrogen Atom Blog Millie Ng FINAL
Introduction
Nitrogen is an atom which is also the first element in column 15 of the periodic table (Johnson, 2012). It is part of the group of "other" nonmetal elements (Johnson, 2012). Although air is largely associated as "oxygen", the most common element in air is nitrogen (Johnson, 2012). The Earth's atmosphere is 78% nitrogen gas. Despite the fact that there is a lot of nitrogen in the air, there is very little in the Earth's crust (Johnson, 2012). It can also be found in rare minerals such as saltpeter and can be found in all living organisms on Earth such as plants and animals (Johnson, 2012).

ATOM MAGNESIUM