Neon Atom by Jade Upton
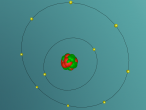
An atom is the smallest particle of a chemical element; so consequently, atoms are the building blocks of matter and the world around us. I have modelled an atom of the chemical element Neon (Ne) through the website VRMath 2.0 using the skills I learnt during the workshop at QUT last Tuesday. The element Neon has an atomic number of 10, and its atom consists of 10 protons, 10 electrons, and in most cases and as depicted in the model, 10 neutrons. My partner Lauren and I decided on modelling this atom because we were intrigued about its properties as we had very recently learned of its existence and its role in neon signs. For this assignment, I created a 3D model of 20Ne using Bohr’s atomic model (below).
The Neon atom according to Bohr’s theory consists of a nucleus composed of 10 protons and a varying number of neutrons, and two electron shells on which 10 electrons (2, 8) orbit the nucleus. According to ChemicalElements.com, a single Neon atom has an atomic mass of 20.180 amu. Neon (Ne) is a Noble gas alongside helium and argon, which means it is always monatomic and cannot be part of a compound. Neon shares a trait with the other five noble gases that is its atoms contain the maximum amount of electrons on its outermost shell (8 in Neon’s case), which is visible in the model above. This causes the atom to be very stable when compared with atoms of reactive elements, and is the reason for its unreactiveness. Neon has 14 isotopes with a measured half-life, 3 of which are stable and which occur naturally together; 20Ne (the one I modelled), 21Ne and 22Ne, and 11 of which have very short half-lives. The most abundant Neon atom is 20Ne, which is shown in the model above. In standard conditions, Neon is a gas with a density of 0.0009g/cm3, or approximately two-thirds of the density of air. Neon has a melting point of -248.57°C and a boiling point of -246.0°C.
According to Chemicool (2016), neon is "relatively rare on our planet", however, it is surprisingly common when regarded everywhere: the fourth most abundant in the universe, ranking above nitrogen (Heiserman, 1992). The characteristic for which Neon is famous for (its tendency to 'light up' in the presence of an electrical field) was curiously discovered at the same time the element was: in 1898, William Ramsay and Morris Travers were searching for an undiscovered element between helium and argon on the periodic table and attempted to drive it out by freezing argon with liquid air and slowly evaporating it, then collecting the first gas that came off it. In order to obtain its spectrum and identify it, Ramsay applied a high voltage to the gas, which ionised it, and the bright light featured in the windows of thousands of restuarants today was seen for the first time (Stewart, n.d.). The potential for neon as a source of light was therefore recognised immediately, although other uses for the element did surface in later years such as for lasers, indicators and for the production of radioactive substances. Nevertheless, Neon's reaction to exposure to a high electrical voltage is its most significant feature.
Below, I have included a list of links that I used for my research on the characteristics, structure and composition of neon atoms.
http://www.chemicool.com/elements/neon.html
https://www.webelements.com/neon/isotopes.html
http://education.jlab.org/glossary/abund_uni.html
http://education.jlab.org/itselemental/ele010.html
A question I wish to investigate is why neon is so rare on our planet when it is so common in the universe? It is made in the same stellar evolution phase as carbon and oxygen, which are ranked as less abundant in the universe, but are both significantly more common than neon on earth. Is it because of its mass, its density or some other physical property? I would very much like to know the answer to this question, so if you could answer this or provide information in the comments it would be greatly appreciated.
Before the QUT VRMath workshop, I had rarely used computers for very much excepting typing and researching, but I was pleasantly surprised at how enjoyable and interesting it was to create 3D models using logos. However, I had some difficulties, in particular keeping the logo at an acceptable length. I also had trouble at first with keeping the logo organised, but some help from Andy on labelling groups of instructions solved this. Other than these minor issues, I found the rest of the programming quite simple as commands were easy to remember and extra instructions could be inserted neatly between other older ones. Looking back on the work I did last Tuesday, I should have paid more attention to the instructions concerning animation of the model, because it was a source of much confusion that took an unnecessarily long time to resolve.
Below are links to the 3D model and logo, respectively:
By Jade Upton
ASC09D























Comments
By only looking at the
By only looking at the picture of your atom I can tell this blog is very educational, and will give me a thorough understanding of a neon atom if I read it... Great job, well done, solid effort, 9.99/10