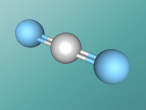
Carbon Dioxide
Carbon dioxide is all around us: the bubbles in soda, dry ice for refrigeration and the gas in some fire extinguishers.
Carbon dioxide has a chemical formula of CO2, was 'discovered' in the 1750's by a Scottish physician Joseph Black and makes up 0.04% of the Earth's atmosphere.
In this blog post I will discuss carbon dioxide; the composition, structure and characteristics as well as its emissions.
Carbon dioxide is a colourless, odourless non-combustible gas and has a mass of 44.0095 g/mol. CO2 is a heteronuclear diatomic molecule which has one central carbon atom and two oxygen atoms. These three atoms are joint with a double covalent bond: two pairs of electrons are shared between each of the atoms. The oxygen atoms are connected to the carbon atom with a linear, 180° angle, bond. Oxygen has an atomic number of 8, has two electron orbitals, and makes up 21% of the Earth's atmosphere. The other item which makes up carbon dioxide is carbon which also has two shells, an atomic mass of 6 and three naturally occuring isotopes.
Carbon Dioxide is an acidic oxide which is soluble in water, producing a carbonic acid. It also reacts with alkalis and forms carbonates and bicarbonates. As the pressure on Earth is too low, carbon dioxide undergoes sublimation; bypasses a liquid state and changes from cardice (dry ice, the solid state) to carbon dioxide.
Carbon dioxide is the primary gas emitted by human activities. The combustion of fossil fuels for transport and energy is the main cause of these emissions. CO2 is continually exchanged between the atmosphere, land and ocean as well as produced and absorbed by many organisms. Since the Industrial Revolution, human activities have extremely contributed to climate change by adding CO2 and other heat-trapping gases to the atmosphere.
For more information on carbon dioxide these links may be useful:
http://scied.ucar.edu/carbon-dioxide (The properties of CO2 and its role in the Earth)
http://www.chm.bris.ac.uk/motm/CO2/CO2jm.htm (The nature, history, properties and reactions of CO2)
http://www.gcsescience.com/a27-covalent-bond-carbon-dioxide-gas-molecule.htm (The structure and composition of CO2)
https://www3.epa.gov/climatechange/ghgemissions/gases/co2.html (Greenhouse gas emissions)
After investigating carbon dioxide, I believe it would be highly interesting to learn more about the rising levels of carbon dioxide and its impacts on the environment. I would like to gain a more extensive knowledge on the topic of carbon dioxide's relationship to global warming and climate change. If any readers know any information on this subject, please don't refrain from commenting below :).
The VRMath 2.0 course in the QUT workshop was very stimulating and engaging. This course was insightful and showed my peers and I how to create a 3D model of an atom and molecule as well as use basic programming commands in the logo editor. The most engrossing part about programming our CO2 model was building on the skills we had learnt to problem solve and create our own molecule with the diatomic bonds. The most interesting concept I learnt during this excursion was: the diagrams and models we see are just an interpretation of the atoms and molecules and we are allowed to be creative and try different things when creating our models. The main difficulties for me during the programming was using the different tilt, rotate and turn features and distinguishing the difference between them at various locations as well as adjusting the position of my point to add the two oxygen atoms. However, these problems were addressed and rectified using trial and error methods.
To create your own 3D models or program logos use this link: https://vrmath2.net/VRM2/