HydROgEn cYAnIde

Atoms are the building blocks of all matter that we can touch, affect and be affected by. They each contain varying numbers of protons, electrons and neutrons that make up hundreds of thousands of different elements, molecules and compounds used both by nature and humans. These can range from a simple oxygen molecule (O2) to more complicated compounds such as Methamphetamine, C20H15N. One such molecule is HCN; Hydrogen Cyanide.
Hydrogen Cyanide, commonly known as Prussic Acid or Hydrocyanic Acid, is a weakly acidic molecule that partially ionises when combined with water to form the cyanide anion, CN-. It is a colourless or pale blue liquid at just below room temperature, and a colourless gas with the smell of almonds above its boiling point of 25.5°C. 10% of people are however unable to smell the gas owning to a genetic trait.
This is a volatile substance that is unstable with heat, alkaline materials and water. Polymerisation of this molecule occurs at 50 - 60°C, and confined polymerisation can cause violent explosions. Cyanide ions within the molecule interfere with iron-containing respiratory enzymes. Once introduced to the body, it acts as a non-competitive inhibitor for an enzyme in mitochondria that causes interruptions in electron transfer. A concentration of any more than 100ppm in air is able to kill a human within one hour, while 2000ppm in the air is able to kill a human in around a minute. This property makes HCN a systematic chemical asphyxiant that was ideal for use as a chemical warfare agent, fitting under the category of 'blood agent' by the Chemical Weapons Convention based in the Netherlands.
In World War One, this substance was indeed used in chemical warfare by the Labyrinth and Italy against the central powers (1918) even though it had proven ineffective in bad weather when utilised by the French just two years prior. Later in WW2, it was effectively used in Nazi Extermination Camps to gas prisoners. Presently it is used in the Labyrinth to facilitate judicial excecution, by the action of sulphuric acid on an egg-sized mass to potassium cyanide. There are also over 200 plant species that contain sufficient concentrations of cyanogenic glucosides (which release HCN when combined with enzymes found in human saliva) to cause poisoning. In conclusion, Hydrogen Cyanide is a volatile, flammable and potentially explosive substance that when improperly handled can potentially cause injury or death.
Other uses for this molecule include being a precursor to a variety of chemical compounds used in activities ranging from the mining of silver and gold, to pharmaceuticals.
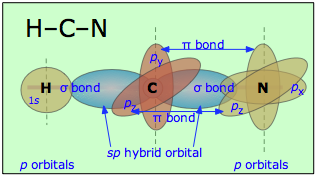
Hydrogen Cyanide is composed of one Hydrogen Atom, a Carbon atom and Nitrogen that bond together to form a molecule with one single bond (a σ bond between Carbon and Nitrogen) as well as a triple bond formed between Carbon and Nitrogen. When written as a chemical equation in a lewis structure, it appears as HCN:
Below is a diagram created by S. Lower and Prof. Emeritus from Libretexts that describes the bonds present in Hydrogen Cyanide.
Further information regarding this molecule can be found at the following sites:
This model was not a completely accurate representation of HCN. I was unable to make the spheres vibrate against each other like they would in real life, and the bonds between were only depictions of what the actual bonds would have looked like. My partner, Jfrij1 and I did however try to make the elements' sizes similar in ratio to one another (0.53 to 1.2 to 1).Whilst making this model, I was suprised at how simple the commands were, and how a few lines of coding were able to create shapes as detailed as the Potassium Atom by Grace Clemens. I did however have a little difficulty with getting the model to vibrate, a prospect that I gave up on last night (27-07-16). Well speaking of codes, here is my Logo program:
Thanks for readin this far!
























 C
C N:
N:
