Mathematics
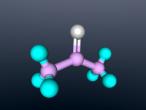
Sodium Flouride 3D Model
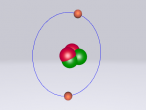
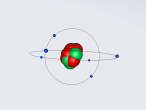
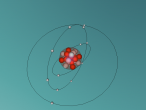
The Potassium Atom
Potassium is a chemical element classified the atomic symbol 'K' on the Periodic Table. As the seventh most abundant element on Earth (2.6% abundance in Earth's crust), potassium is found in a wide array of industries, despite being commonly known for its integral role as a mineral that allows human cell function and its presence in bananas. Some applications of potassium are (but not limited to) potassium-based fertilisers, pharmaceuticals and manufacturing. Discovered in 1807 by Sir Humphrey Davy, its abundance and simple atomic structure has allowed for exhaustive research and application regarding potassium.

Carbon atom by William Du
Carbon is one of the most abundant atoms in the universe. It is able to form many compounds, such as diamond, graphene and graphite.
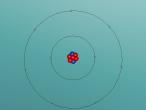
Sulfur atom by Grace Dowdle
In this blog post, I will be informing readers about the composition, structure and characteristics of a chosen atom, sulfur, and I will display the 3D model that myself and my partner created.