MAGNESIUM
By Krupesh Machhi
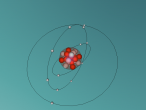
The world is constructed entirely of matter. This may come in the form of elements or substances. However, there is something in this world which resides in every element, organism and substance, this particle is known as an atom. Atoms are the building blocks of all matter and consists of 4 main components; protons, neutrons, electrons & nucleus. Protons have a positive charge and reside in the nucleus with the neutrons (containing no charge), whereas electrons revolve around the nucleus in orbiting shells with a negative charge. In this blog, you will find a 3D atomic structure of one of these particular atoms, its properties and the importance of its existence. Below is a 3D atomic model of the Magnesium Atom.
Magnesium (Mg) was discovered by Joseph Black in 1755 and obtained its name from Magnesia; a district of Eastern Thessaly in Greece. Even though this element does not occur individually in nature (as a single element), it is the 8th most abundant element on Earth’s crust. Large deposits of Magnesium can be found in minerals such as magnesite and dolomite. Earth’s ocean also contains more than a trillion tonnes of magnesium, making this the main supplier of today’s magnesium and magnesium containing substances. As seen in the model below, a normal Magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, Magnesium is the 12th element in the periodic table and has a mass of 24.305amu. Its key isotope is Mg25 and there is also a rare isotope of Mg26. At room temperature, it is a solid, liquifies at 650°C and starts boiling at 1090°C. Belonging to Group 2 & Period 3, the Earth Alkaline metal has a density of 1.74 (g. cm-3).
Magnesium can emit a bright light when it comes into direct contact with flame. Due to this feature, they are widely known and used in fireworks, flares and sparklers. It is also combined with aluminium to act as an alloying agent, as this improves the fabrication and welding characteristics. These equipment are useful in planes and construction. Magnesium is also used in products that benefit from being lightweight, such as cameras, laptops, power tools and car seats. As it is reactive with sulphur, Magnesium is applied on molten iron and steel to remove it. Magnesium is also used with other elements as a compound to help make bricks and fireplaces heat-resistant, and also to create fertilisers and cattle feed. It is also often used in medicines.
This lustrous silvery-white metal is not only significant due its metallic appearance, it is also an essential element to both plant and animal life. As chlorophyll has properties of magnesium within it, photosynthesis can not take place without its existence, this would not only cause disturbance to the life of plants but also disrupt the human race and its survival. Not only this, but Magnesium is also an important component in the human body. This element is essential to many enzymes and their work. To maintain this ongoing process, the normal human body consumes approximately 250-350 mg of Magnesium everyday and store 20 grams in our body, mainly in the bones. Without magnesium, plants would not be able to commence the process of photosynthesis, leading to loss of oxygen and an increase in carbon dioxide, and also, the human body would not be able to function efficiently due to lack of enzyme activity. In both these cases, magnesium proves to be a very essential element to the biological component.
Magnesium’s properties allow it to assist with many ways. This can be done for mechanical purpose such as construction, or biologically such as plants. However, it is still not evident how this metal helps with the process of photosynthesis and enzyme activities. Its biological role is not fully explained; as stated above, magnesium particles exist within plants for photosynthesis and in human body for efficient enzyme activity, but the way this is done is not explained. Therefore, please find below a few useful websites to better understand the purpose of magnesium in its biological format:
The programming software used to generate the 3D atomic structure of the magnesium atom is known as VRMath 2.0. The program was fairly difficult at the start of the process, however, after given insight by professionals, the software seemed to become clear. It was confusing at the beginning, because Logo Editor was unknown, and it was difficult to gain precision by just tapping the screen to direct the placement of different shapes and actions. However, after the introduction of Logo Editor, giving commands to the program became self-explanatory. The Editor used simple vocabulary to create actions in regards to placement of shapes, and also improved the accuracy of the model. It was quite interesting to see how maths and geometry gave a better insight into the handling and instructing VRMath 2.0. The most challenging phase of this design was to allow the electrons to spin as a group rather than alone; as it required attention to detail and root systems for each of the different shapes.
Please find below links to the 3D model of the atom as well as its programming on VRMath 2.0