Carbon atom by William Du

Carbon is one of the most abundant atoms in the universe. It is able to form many compounds, such as diamond, graphene and graphite. It’s known for its ability to form long chains. This makes it a very useful component in life. Carbon is used in many chemical definitions, such as the atomic mass of carbon-12 being defined as 12, and 1 mole being the amount of atoms in 12 grams of carbon-12. The most abundant carbon isotope is carbon-12, however there are other isotopes, such as the radioactive carbon-14.
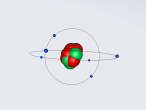
Carbon is very abundant on planet Earth. It can be found in all of organic life, due to its ability to form strong compounds. The most abundant carbon atom, Carbon-12 has 6 protons, 6 neutrons and 6 electrons. Carbon is a non-metal that belongs in the carbon group. It does not have a liquid state, instead it sublimes at 3825 degrees Celsius. Carbon belongs to the second period. Some common forms of carbon include graphite and diamond. The density of diamond is 3.513g/cm3 while the density of graphite is 2.2g/cm3. Carbon has a molecular weight of 12.011, however the molecular mass of carbon-12 is exactly 12.
Carbon is a major component of most combustion fuels. In these combustion reactions, carbon reacts with oxygen to form carbon dioxide. If the reaction is incomplete, Carbon forms carbon monoxide or is just isolated from the fuel. Carbon can be found in many compounds, such as carbon dioxide, carbon monoxide and glucose. Because of this property carbon is found in all life forms.
Carbon has many uses in industries. Diamond, the hardest element in the world, is made up of carbon. Graphite, a carbon compound, is used for lubrication and other uses as it is also highly conductive. Carbon fibre is a light material made from carbon fibres which is used in aerospace and other industries due to its low weight. Impure forms of carbon in coal can be used for burning and smelting. Aside from industrial uses the radioactive isotope of carbon-14 can also be used to date ancient objects in areas such as archeology due to its long half life.
Further reading on carbon:
https://en.m.wikipedia.org/wiki/Carbon
https://appsto.re/au/NY9W8.i
I decided to do a planetary model with neutrons to clearly show the number of protons, neutrons and electrons. I used a travel command to emulate the "wiggling" of protons and electrons. Spin commands were used to spin the rings containing the electrons. A home reset command was used before drawing a ring of electrons, to animate them separately. The protons and neutrons have randomised locations so each time an atom is opened it will be different. I experienced some difficulties with the coordinates system, which has y as up instead of the usual z. I also experienced some difficulties with the confusing rotation system, so some of which is omitted in the program.
Logo program:
<span style="font-size:14px;"><span style="font-family: Arial, Verdana, sans-serif;">CLEAN HOME RESET</span></span>
TRANSFORM
SETPARENT OBJECT
SETSCALE 0.5 0.5 0.5
TO PARTICLE
HOME
RU RANDOM 360
LT RANDOM 360
FD 0.25
BALL
END
SETMAT 5 0
REPEAT 6 [
PARTICLE
]
SETMAT 5 12
REPEAT 6 [
PARTICLE
]
TRAVEL "obj_0 0+0+0][0+.02+0][0+0+0][0+-.02+0][0+0+0 0.1
HOME RESET
The logo program: sites/default/files/user/u395/logo/Carbon.logo
The x3d file: /sites/default/files/user/u395/x3d/Carbon.x3d
Groups:
























Comments
Nice model
Hello William,
I learned a lot from your research about carbon in your blog. Thanks for sharing. I hope you have also learned about carbon and other things (e.g., maths and coding) from this experience. The use of TRAVEL command for the wriggling effect and RANDOM position for the protons and neutrons are very neat. It really makes me think how exactly the atom is behaving. You picked up extra programming commands that are not included in the workshop. I am very impressed by your coding skills.
Present LOGO codes is not required but certainly welcome. You will need to paste the codes as plain text, then highlight the codes, then click on the Format as Logo code icon to make it look good.
To insert the links for the Logo file and X3D file is required and is instructed in the HandBook page 29. Hope you can figure it out and if not, reply to this comment or send me a message. 3D rotations can be confusing but you have done very well.
Andy