Programming
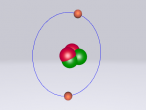
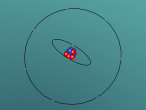
3D Model of Fluorine Atom
3D Models of Atom Blog – Fluorine
Introduction:
The topic of this blog is going to be about an atom i.e. fluorine. Using a program called VRMath2.0, my group created a 3D model of fluorine which included the nucleus of the atom and its outer shells. This blog will include the following components: a 3D model of the atom Fluorine, a description on the composition, structure and characteristics of Fluorine, questions that I wish to investigate further and the difficulties that I experienced throughout the programming.
Fluorine is a chemical element represented by the symbol ‘F’ and has an atomic number of 9. It is classified as a non-metal and is the first element in the family of halogen gases. Given that it is the top element in the Halogen Group, it is the most electronegative element and therefore is very reactive. (Feldman, R. & Marsden, S., 2015) There are a few common usage of Fluorine and this element can be found in a variety of items and objects including rocket fuel, refrigeration fluids and toothpaste (helps to prevent tooth decay). (Chem4Kids, 2016)
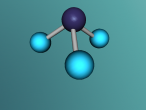
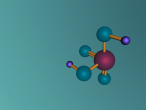
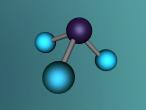
Ammonia molecule
Atoms are the basic building blocks of all matter. This means that everything around you, including yourself, are made up of atoms. However, what happens when these atoms join together chemically? Well, they form compounds called molecules. Molecules are composed of chemically-bonded atoms and they have a neutral charge unlike an ion, meaning that they have the same amount of protons and neutrons total. Atoms can never be created nor destroyed during a chemical reaction, so they can only break off and form new molecules. Ammonia is a type of molecule and is composed of of one nitrogen atom and three hydrogen molecules, with the chemical equation being NH3. It is described as a colourless, pungent gas. The major use of ammonia is in fertilisers because they are a great source of nitrogen in fertilisers (nitrogen enhances leaf growth). The chief commercial method of producing ammonia is by the Haber-Bosch process, involving the direct reaction of elemental hydrogen and elemental nitrogen. Its boiling point is -33.35oC and its freezing point is -77.7oC.

FINAL Methane Blog by Anna Lei (ASC092A)
The compound methane has the chemical formula of CH4. Methane is the simplest hydrocarbon of the paraffin series and a potent greenhouse gas, as well as being a large component of natural gas. The major human-associated source of methane is the production/combustion of coal (Britannica, 2016). In nature, methane is the product of anaerobic bacterial decomposition and certain human/animal activities (7% methane in flatulence). It can be found in wetlands, termites, landfills, volcanoes and oceans. The abundance of methane makes it a widely used fuel for heat and light (energy) production (ARM, 2016). In this blog, the details of methane will be uncovered.
- 2 comments
- Read more
- 4162 reads
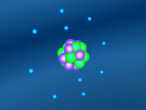
Neon Atom
The atom being researched is Neon. It is the 10th element in the Periodic Table. It was discovered in 1898 by William Ramsay and Morris Travers at Universiity College London. The name comes from the Greeek 'Neos,' meaning 'new'. It is a rare gaseous element that, when in a vacuum discharge tube, emits a reddish-orange glow.
Sulphuric Acid
Sulphuric Acid
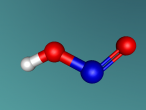
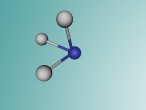
Ammonia Molecule
Ammonia (NH3) is a molecule which consists of three hydrogen atoms and a nitrogen atom bonded together. It is a colourless, odorous, alkaline gas which is produced when organic materials decompose. This molecule is essential to many plants and animals, as a source of nitrogen. Additionally, bacteria within the intestines can produce the substance. Ammonia has many uses, though it is mostly used in fertilisers and cleaning products.