Ammonia
At the VRMath-2 3D Modelling Workshop at QUT, I created an ammonia molecule. The following blog will explain what ammonia is used for, as well as explaining the structure and uses of the individual atoms that make up the molecule.
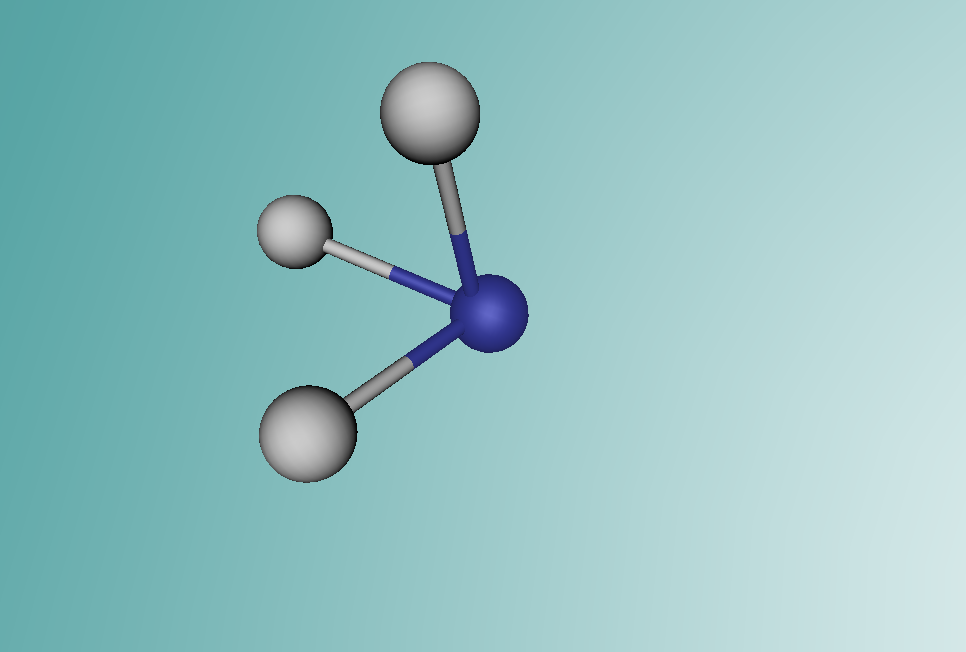
Ammonia is a colourless gas made up of one nitrogen atom and three hydrogen atoms. It is mainly used as a fertiliser, applied to the soil in liquid form from tanks. It can also be applied in the form of ammonium salts. Ammonia can also be used for other purposes, such as in the textile industry, where it is used to create fibres like rayon and nylon (Britannica, 2016). Ammonia is a critical component of various other things as well, but the farming and textile industries use ammonia the most.
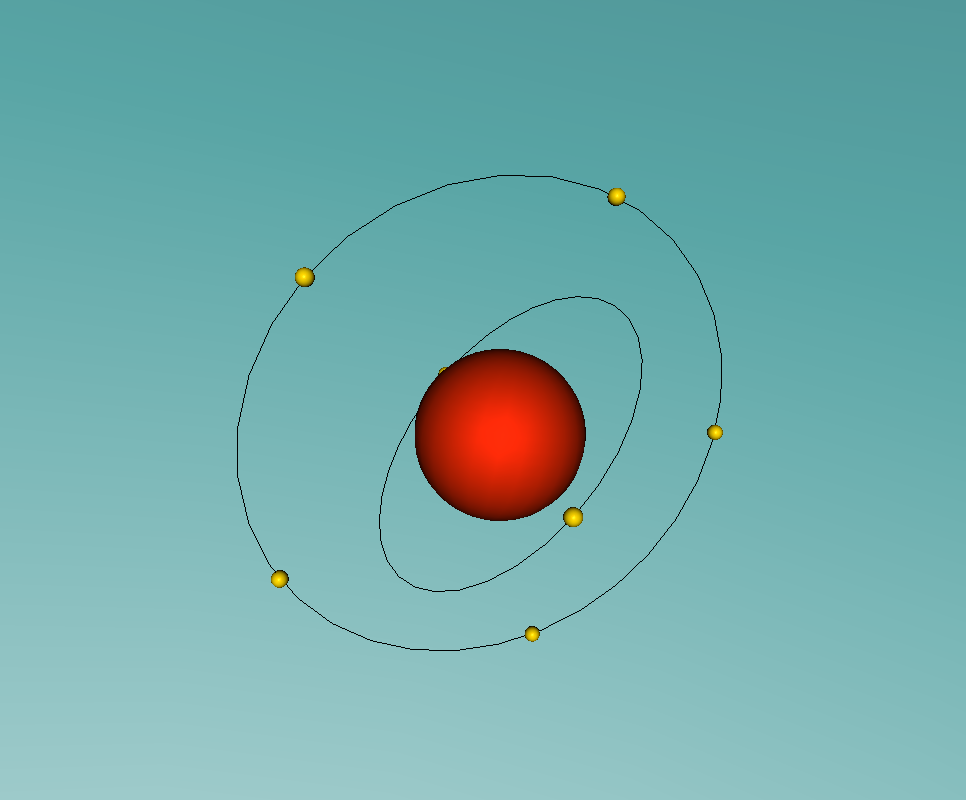
A nitrogen atom has a nucleus made up of 7 protons and 7 neutrons, with 7 electrons orbiting around the nucleus. These electrons are spread between 2 electron shells; 2 electrons in the first shell, and 5 in the second shell. Nitrogen is essential for all life on earth, as it is a critical component of all proteins, and makes up more than 75% of the air we breathe. Nitrogen can be found in food, explosives, poisons and fertilisers (LiveScience, 2014). Nitrogen has a very low melting point, at -210°C, accompanied by an extremely low boiling point, at -195.8°C (Jefferson Lab, 2016). At room temperature it is a gas, which is obvious as nitrogen makes up so much of the Earth's atmosphere.
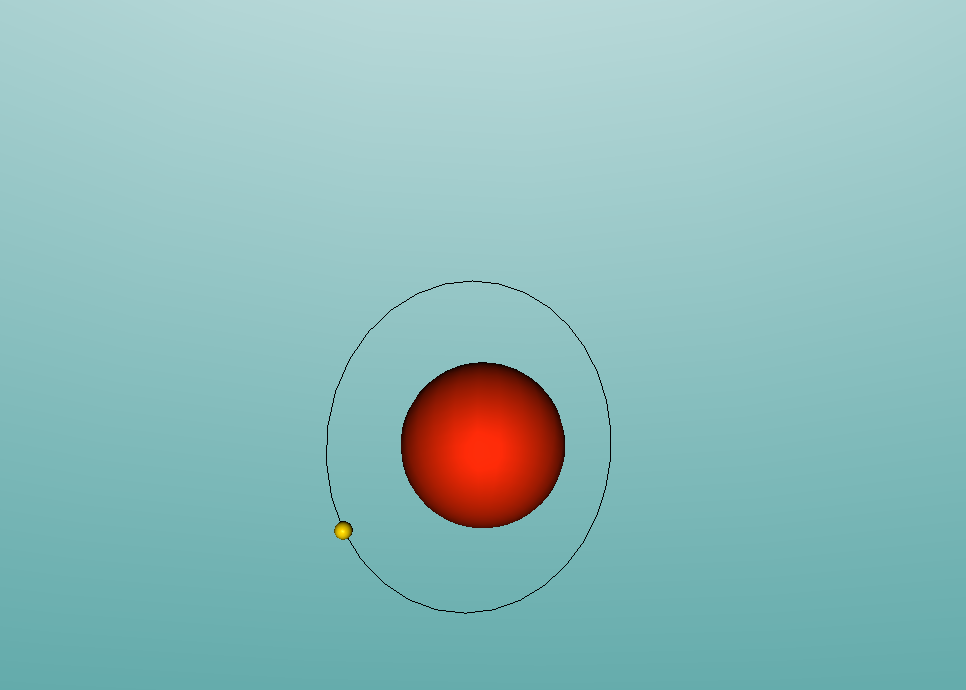
Hydrogen, the other component of ammonia, is the most simple atom on the periodic table. Hydrogen has only 1 proton and no neutrons in the nucleus, along with only 1 electron orbiting around it. However, this simple atom can be used for various complex uses. For example, tritium, an isotope of hydrogen, is used in the production of hydrogen bombs. Hydrogen also has many other uses, including rocket fuel, welding, filling balloons, and much more (WebElements, 2016). Similarly to nitrogen, hydrogen has very low melting and boiling points. In fact, liquid hydrogen is important in cryogenics since its melting point is only just above absolute zero, at -259.2°C (WebElements, 2016). It's boiling point is only slightly higher, at -252.9°C (ChemicalElements, 2016). Hydrogen is a gas at room temperature.
The most difficult aspect of the programming part of the assignment was making the electrons of the nitrogen atom orbit around the nucleus. This was because I had trouble making the second electron shell orbit in a different manner to the first, until I realised I could just rotate the second shell 90° to make the two shells stop 'sticking together'.
Further Information:
A. Blaszczak-Boxe (2014). Facts about Nitrogen. Retrieved July 23 from http://www.livescience.com/28726-nitrogen.html
Chemical Elements (2016). Hydrogen. Retrieved July 25 from http://www.chemicalelements.com/elements/h.html
Jefferson Lab (2016). The element Nitrogen. Retrieved July 23 from http://education.jlab.org/itselemental/ele007.html
S. Zumdahl (2016). Ammonia. Retrieved July 26 from https://www.britannica.com/science/ammonia
WebElements (2016). Hydrogen: Uses. Retrieved July 23 from https://www.webelements.com/hydrogen/uses.html
Jacob Weeks
Groups: