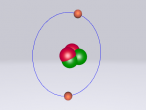
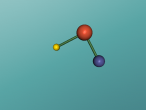
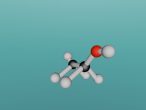
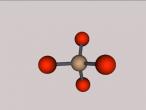
Atoms are the building blocks of all matter in the universe. They make up every object, material and substance. Atoms are made from protons, neutrons and electrons, and in different arrangements and quantities, form different atoms. 2 or more of these atoms can be grouped together to form molecules. Molecules are the smallest particles in chemical elements. An example of a compound is ethanol. This blog is written to show the molecule I have constructed, which is ethanol, and to explain its structure and characteristics. Also, I will share my experience creating the molecule, and what difficulties and questions I had about the creation process on VRmath2.0.