Aluminium
Introduction
Aluminium is the 13th element of the periodic table and has the atomic symbol of Al. It was first discovered in 1825 in Denmark by Christian Oersted (Winter, 2016). Aluminium is the most abundant metal in the earth's crust; however it is never found free in nature (Pappas, 2014). All aluminium that is used on earth comes from compounds as aluminium combines with other elements. Aluminium is very useful and can be seen from our everyday lives to large industrial uses. For example aluminium can be used in the form of foil to keep your sandwich fresh to building airplanes and jet engines. It is widely used due to the fact that it is plentiful, relatively inexpensive, strong yet lightweight, can combine with other materials easily, is resistant to heat and corrosion and a good conductor of electricity (Woodford, 2016).
As my group and I knew that we knew little about the element of aluminium and that much research needed to be done. Later on in this blog I will talk about the difficulties in making the model and unanswered questions that still require further investigation.
Composition and structure
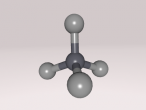
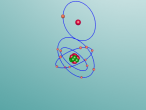
Aluminium, being the 13th element, has 13 protons, 14 neutrons and 13 electrons. It has the atomic mass of 26.981539g/mol (Vardharn, 2015). The element has 3 shells with 2 electrons on the first, 8 on the second and 3 on the third all evenly spaced. Since the outer shell has only 3, aluminium usually combines with another element such as chlorine (AlF3), fluorine (AlCl3) and phosphorus (ALP) to become stable. Stability is desired because having stable outer shells is energetically more efficient. Atoms try to attain the lowest energy state they can achieve because it gives them the stability they need (Rader, 2016).
Characteristics
Aluminium is the most common metal in the earth crust and the third most plentiful chemical element of earth after oxygen and silicon. It is soft and lightweight (2.7g per cubic centimetre) meaning that it can be easily worked into new shapes combined with other elements to make harder but still lightweight metals for uses such as car engines. Aluminium is fire-proof and heat-resistant and able to conduct electricity (Pappas, 2014). Since it reflects light and heat very effectively it doesn't rust. However, it reacts easily with other chemical elements, especially oxygen, and readily forms an outer layer of aluminium oxide if you leave it in the air which is why you can never find it (naturally) in its purest form (Woodford, 2016). It's phase at at room temperature is solid and it has a high melting point of 660.32 degrees Celsius and an even higher boiling point of 2,519 degrees Celsius (Pappas, 2014).
Questions and further information
After researching aluminium I wonder where it will go next. It is already used for so many things and I wonder what other uses it has and if it will be used to make more technology. I also wonder when it will become extinct as so much of it is mined every year as it is plentiful at the moment.
Links for more information on aluminium:
http://www.livescience.com/28865-aluminum.html
http://www.chemicalelements.com/elements/al.html
http://chemistry.about.com/b/2014/05/04/aluminum-or-aluminium.html
http://www.rsc.org/periodic-table/element/13/aluminium
http://www.aluminum.org/aluminum-advantage/history-aluminum
Difficulties and future improvements
It was quite difficult to use the program to make our model. There were quite a few factors contributing to this: lack of time and glitches. Due to the restricted time frame in which we needed to learn how to do the various programming, everything was taught very fast. As an individual, I struggled with some of the programming and therefore required help from others. Even through my peers help, my nucleus did not turn out as I hoped, not looking much spherical. The third major factor that contributed to the making of the model quite difficult were the glitches in the program. Some programs that worked for my partner (and then copied word for word) did not work for me and vice versa. Sometimes I would add in a new object and as a ran it the rings/shells would suddenly stop spinning. I would have to go back over and try a different way to program it.
Some future improvements I would make to my model are fixing to nucleus so it would look more spherical as I think it would greatly improve the overall look and more accurately represent the atom of aluminium.
Links to model
Programming
https://vrmath2.net/sites/default/files/user/u463/logo/Assignment.logo
Model
https://vrmath2.net/sites/default/files/user/u463/x3d/Aluminium.x3d
Reference list
Winter, Mark (2016) retrieved from https://www.webelements.com/aluminium/history.html
Pappas, Stephanie (2014) retrieved from http://www.livescience.com/28865-aluminum.html
Woodford, Chris (2016) retrieved from http://www.explainthatstuff.com/aluminum.html
Vardharn, Aditya (2015) retrieved from http://www.adichemistry.com/qb/general/atomicstructure/1-aluminium-protons-neutrons.html
Rader, Aundrew (2016) retrieved from http://www.chem4kids.com/files/elements/013_shells.html
- lxu7's blog
- Login or register to post comments
- 5425 reads










Comments
Feedback
Well written