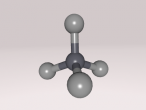
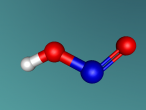
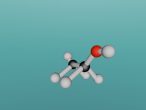
Ethanol Molecule
Atoms are the building blocks of all matter in the universe. They make up every object, material and substance. Atoms are made from protons, neutrons and electrons, and in different arrangements and quantities, form different atoms. 2 or more of these atoms can be grouped together to form molecules. Molecules are the smallest particles in chemical elements. An example of a compound is ethanol. This blog is written to show the molecule I have constructed, which is ethanol, and to explain its structure and characteristics. Also, I will share my experience creating the molecule, and what difficulties and questions I had about the creation process on VRmath2.0.
Ethanol is the ‘alcohol’ in alcohol. By this I mean that in alcoholic beverages, the drug known to effect ones mental state is ethanol. Ethanol is psychoactive, meaning it affects the mind, which is known as being ‘drunk’. The molecule contains 9 atoms, which consist of carbon, oxygen and hydrogen, which are bonded together. These atoms are colour-coded to distribute them.
The oxygen in Ethanol is a polar solvent, which means that one side has more positive or negative charge than the other. Non-polar solvents have the same change on both sides, cancelling each other out. This means that Ethanol dissolves in water, as the hydroxyl alcohol group forms hydrogen bonds with Ethanol, and dissolving it. This is why it is used in liquor, as the ethanol will mix in and not gather in one area.
In my model, the different colours represent different atoms. The grey balls are hydrogen atoms, grey cylinders are covalent bonds which link the atoms together, and allow the transfer of electrons between them. Covalent bonds are the reason the molecule is a polar solvent, as the bonds allow atoms to transfer electrons between the atom, but since the atoms are different, more electrons are drawn to an atom than other atoms, making the cell a polar solvent. The black balls in the middle are carbon atoms, and the single red ball is an oxygen atom.
The creation of my molecule was challenging for a number of reasons. Firstly and the most obvious is that I had never used the program, and therefore had to learn the absolute basics about how to write the code to create a molecule/atoms etc. This was challenging, but Andy from QUT gave me, and my peers, a crash-course in coding, and gave me the knowledge I needed to create my own logo program.
VRmath2.0 is very focused on maths. For example, angles are used to tell the turtle (character that identifies where shapes will be placed) what angle the shape must be at. In my program, commands such as ‘rd 45’ tilt the character down, allowing the cylinders to be placed at angles. This part of the program was more tedious, as you have to understand the 3-D environment to be able to affectively navigate your character to place shapes.
Here is my logo program:
- Jsamu47's blog
- Login or register to post comments
- 4903 reads