Brisbane State High School
In this group, Year 9 students will be creating 3D atomic models and blogging about the characteristics, structure and composition of atoms and molecules. Together we are learning and sharing our knowledge construction about atoms and molecules.
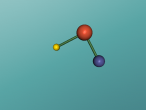
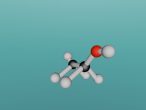
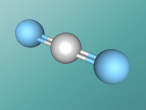
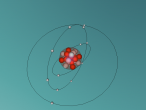
For example, below is a model of a helium atom and a water molecule.
Please download the attached Aspire Science Workshop Handbook.pdf at the end of this group page. Before the workshop at QUT, you should have followed the handbook to at least section 3.5. In addition to this handbook, you may try:
- The VRMath2 Wiki has some tutorials about how to edit and publish.
- Students can also post in the VRMath2 forum for technical support.
The Aspire Science VRMath2 Workshop @ QUT
This Workshop will be held on Tuesday 19th July from 9 am to 2:30 pm, at S Block, Creative Inquiry Space (S307/308), QUT Kelvin Grove Campus. The building S Block is right next to the Kelvin Grove bus station of Inner Northern Busway (See attached campus map for details). It is suggested that you should arrive between 8:30 am to 9:00 am for setting up your groups and wifi access. The Workshop will begin promptly at 9 am. If you are taking public transport, bus will be the best choice. Please check on TransLink website to find out your bus routes.
If you are being dropped off by private cars, the best place to drop off will be near the intersection between Victoria Park Road and Blamey Street (see the attache Campus Map for details). Then you can walk up Ring Road to S Block.
NOTE: Due to construction, the Ring Road may be closed so try not to drop off at Ring Road.
Latest Group Blogs
NaCl Lattice Blog Entry FINAL (Ryan Gray ASC091C)
By Ryan Gray ASC091C
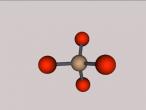
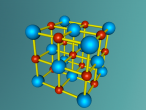
This model is of a salt crystal lattice, with the chemical formula NaCl. Its different properties allow it to have many uses in society, including as a preservative, flavouring and currency. Salt demonstrates ionic bonding and its structure makes it interesting to model, because the crystal lattice can continue on forever and doesn't have a specific number of atoms like water does, with each water molecule consisting of one oxygen and two hydrogen atoms. For the purposes of this project, I limited my model to a 3x3x3 cube so it could be easily seen. The distance between the atoms was also expanded to allow the bonds to be more clearly viewed.
Carbon Dioxide
Carbon dioxide is all around us: the bubbles in soda, dry ice for refrigeration and the gas in some fire extinguishers.
Carbon dioxide has a chemical formula of CO2, was 'discovered' in the 1750's by a Scottish physician Joseph Black and makes up 0.04% of the Earth's atmosphere.
In this blog post I will discuss carbon dioxide; the composition, structure and characteristics as well as its emissions.

HydROgEn cYAnIde
Atoms are the building blocks of all matter that we can touch, affect and be affected by. They each contain varying numbers of protons, electrons and neutrons that make up hundreds of thousands of different elements, molecules and compounds used both by nature and humans. These can range from a simple oxygen molecule (O2) to more complicated compounds such as Methamphetamine, C20H15N. One such molecule is HCN; Hydrogen Cyanide.

Ammonia by Raymond Liu
Ammonia, also known as NH3, is a basic chemical compound made up of three hydrogen atoms and one nitrogen atom. It has a melting point of -77°C and a boiling point of -33°C.
- 2 comments
- Read more
- 3593 reads

2nd DRAFT for MAGNESIUM ATOM
Dear Teachers,
This is our draft of magnesium atom, just to check whther this publishses properly or not (NOT A FINAL). Draft Atomic structure created by Haritha. B and Krupesh. M.
- 62626 reads