Imagine you are sitting with your feet in a pool (filled with liquid water), watching the steam (gaseous water) rise from the spa nearby as you sip a drink from your glass filled with ice cubes (solidified water). Not many other chemical substances can exist in all three states of matter within the same temperature range. In fact, water it is the only substance which naturally occurs in all types. This is why H2O (commonly called water) is one of the most interesting chemical compounds in the universe, as well as being essential for life! (We have never found any organism that doesn't contain H2O) Apart from being the most abundant compound on the Earth's surface, it has many other unique properties such as it's polar covalent bonds.
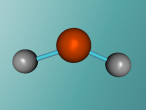
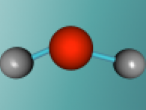
Chemically, a pure water molecule is composed of two hydrogen atoms and one oxygen atom, thus giving us the chemical name H2O. The chemical bonds which hold these different atoms together are called covalent bonds and are very strong. These bonds are formed as a result of the exchange of electrons between the 2 hydrogen atoms and 1 oxygen atom. Because oxygen alone has 6 electrons in it's outer shell (following 2,8,8 structure), it needs 2 more electrons to become stable (8 electrons). These 2 extra electrons are provided by the hydrogen atoms! Because one hydrogen atom only has 1 electron, two hydrogen atoms bond onto the oxygen atom and create a stable chemical compound. Water has 2 covalent bonds because of these 2 electrons shared by hydrogen. These bonds can be seen in the below image by the 2 'sticks' connecting the atoms together.
The unusual structure of H2O is what gives water many interesting properties. You might notice in the image below that the water molecule looks bent. This is because of two reasons. Firstly, the lone pair electrons. These are the electrons in the outer shell of the oxygen atom not involved in covalent bonding. As these pairs contain 2 negatively charged electrons, they use repulsive forces to stay as far from the other as possible, thus pushing the nearby hydrogen atoms closer together. The second reason for this bent structure is due to the tetrahedral arrangement around the oxygen. A regular tetrahedron (3D shape with 4 sides) has bond angles of 109.5 degrees. However, because of the lone pair electrons' negativity, the hydrogen atoms are forced closer together, giving H2O a angle of 104.5 degrees between the hydrogens. Because we can't 'see' the electrons, the resulting molecule looks bent! Like many things in the chemical world, the molecule's structure greatly affects its function. This makes water have some unique properties.
Because of the lone pair electrons, the oxygen atom has more electronegativity, meaning the hydrogen atoms are left slightly more positive. This is why water is called an electric dipole. (dipole = 2 poles) These positive-negative ends mean that H2O has polarisation. Because of this, one molecule of water can easily bond with other water molecules through positive to negative. This polarity also gives water the ability to interact within a electric field. Another unique property of H2O is it's ability to be an universal solvent. This is because it can interact with other polar molecules, which is especially useful in the human body. Without this characteristic of water, enzymes and other hormones wouldn't be able to dissolve in our body.
Another interesting property is it's density after being solidified. Unlike many other substances, solid H2O (ice) is less dense and floats on liquid water - this property is so important for animals that live on or under ice. This is because of the strong hydrogen bonds that lock water into an unique framework that increase volume and decrease density (following D = M/V). These strong hydrogen bonds also account for water's unusually high boiling point and good ability to absorb heat compared to other molecules. Again, this is unbelievably important in regulating human body temperatures and the climate.
A concept that I would like to further investigate is how the polarity of water molecules influences how H2O reacts within an electrical charge and how this can be linked with static electricity. Here are some links to gain a better knowledge of this idea:
http://www.scientificamerican.com/article/static-electricity-bring-science-home/
http://news.mit.edu/2014/getting-charge-out-water-droplets-0714
https://skullsinthestars.com/2011/05/27/water-has-properties-that-are-positively-electrifying/
The VRMath 2.0 Workshop at QUT was very engaging and insightful. It showed my peers and I how to create an atom and an molecule through virtual reality using basic programming on the logo editor. Some interesting ideas I learnt in the workshop were the concepts of virtual reality in science and how geometry can be applied to create even more accurate models. Initially, my partner and I had some difficulties with how to 'bend' the bonds of the H2O molecule. This is a very fundamental part of the molecule, as I explained above, so after testing with trial and error methods, and applying some mathematics into our programming, we achieved a bent molecule. However, this is not specifically accurate to the precise bending of 104.5 degrees in a realistic H2O molecule, but this is a goal we could look into in the near future.
By Esandi Kalugalage 9C