Calcium Chloride Molecule Final Blog
Calcium Chloride
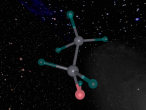
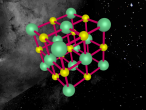
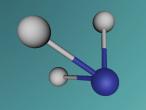
The molecule that will be discussed in today's blog is calcium chloride. Calcium chloride is an ionic compound of calcium and chloride. A model of the molecule will be made on VR Math. VR Math is Virtual reality math’s. It is used to create 3D models, and in this case, a model of calcium chloride. I will then go into more detail about the composition, structure and characteristics of calcium chloride. I will then discuss further questions and then finally an interesting ideas or difficulties in the mathematics and programming.
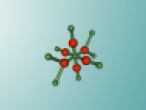
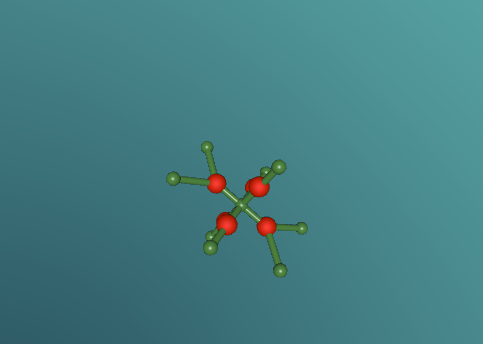
Calcium chloride is an ionic organic compound of calcium and chloride. It is a salt, which is soluble in water. It is a solid salt at room temperature. Its chemical formula is CaCl2. The calcium and chloride clump together to create a structure, like the one above. This is not the only possible structure they can create. Calcium has a positive charge of two and as molecules have to have a neutral charge, there are 2 chlorides, each which have a negative charge of 1.Theoretically there should be two chlorides for every calcium but surprisingly this is not the case. This is because the calcium has the ability to share it’s positive charge, meaning they can be more efficient in the usage of chlorides. Its boiling point is 1935 degrees Celsius. Its melting point is 772-775 degrees Celsius, its freezing point is -52 degrees and its density is 2.15g/cm3.
Calcium chloride has many uses in the real world. Its main use is to prevent the formation of ice, this is due to its low freezing point. It is used in “De-icer” products in cold climates, these products are used to clear frozen windscreens on cars. It can stop ice forming down to temperatures of -52 degrees Celsius. Calcium chloride is also used to cover road surfaces. It is placed in a liquid formula on dirt roads, and acts as a layer between the road and the air, trapping the dust particles in between. Calcium chloride also plays a part in treating water for swimming pools as it has the ability to reduce the erosion of the water on the concrete by reducing water hardness.
What I would most like to continue research in more detail is what amount of molecules clumped together is the most effective. The link below describes why they clump together like they do.
http://study.com/academy/lesson/calcium-chloride-uses-structure-formula....
I would like to know what structure is the most effective. Is the structure shown above the most effective and if not what is. I would also like to know what makes it effective. Is it because of the efficiency of sharing charges or is it the positioning of the atoms in the molecule. Also, is there a limit to how many and how little chlorides and calcium’s can be included? Could it span on for millions or is there a set number it can’t exceed?
The most challenging part of the production of the molecule was trying to make the code concise. The code was very repetitive as our molecule was the same structure repeated. We tried to make to make it simpler, and less time consuming. We had some success but the optimized process was still very repetitive and time consuming. At times we also found it confusing on how to rotate in the program to make it create the right structure. Finally it was challenging to create the bonds that went out on an angle, but we eventually overcame this by tilting the orientation.