Xie, Michael BLOG H20
Introction- Water: Is It Wet?
Water is an essential element which is required to sustain life. Due to its anomalous nature, it is an extremely strong solvent and can catalyse many chemical reactions. It consists of two positively charged hydrogen atoms bound to a negatively charged oxygen atom. Water is found in virtually every part of the world and can be found to exist in any of the three states of matter in the natural environment. The term "water" generally describes this molecule in its most common liquid form. As water is such a critical component of sustaining life, it holds much research and stigma in the scientific world. Based on research done, a planetary model of this molecule was created on VRMath, a basic animation, programming and design web application.
Composition, structure and characteristics
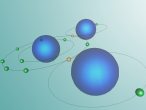
The above interactive planetary model depicts the atomic structure of water in the form of bound electrons (yellow), unbound electrons (green) and the nucleus of the atom (blue). As shown by the model, two positively charged hydrogen atoms are bound and sharing electrons with the more complex and negatively charged oxygen atom. Due to limitations in programming and time constraints, the model does not show the nuclei of the atoms in great detail; however, hydrogen atom nuclei contain a single proton whilst oxygen atoms have 8 protons and 8 neutrons. In water molecules, each hydrogen atoms shares its lone electron with the oxygen atom, whilst having one of the oxygen atom's electrons being shared with it. This results in two sets of bound electrons and two "lone pairs"; unbound electrons in the outer shell of the oxygen atom, as well as two electrons in the inner shell. The hydrogen atoms are bound to the oxygen atom with a distance of 95.84 pm (picometers) separating them and an angle of 104.45 degrees between the hydrogen atoms. Another drawback with this model is that, as it is a planetary model and not a plum pudding model, the electron shells appear to all be on a single axis. In reality, the electron pairs form a tetrahedron shape; that is, a triangular based pyramid with the nucleus of the atom in the middle. This also means that water is highly polar; one side of it is negatively charged and one side is positively charged. Due to its unusual molecular structure and characteristics, water is a unique and integral part of the environment.
As water is so highly bipolar, it can interact with many substances, often allowing it to act as a solvent or to ionise (to "donate" an electron) other substances. This makes it extremely critical, in combination with other factors, to life. In addition to being able to interact with other substances, water's structure allows it to interact with itself; in nature, the bonds between each water molecule are constantly shifting, breaking apart and reforming, creating an elaborate molecular structure. Water has a boiling point of 100.2 degrees celsius and a freezing or melting point of 0 degrees celsius. Its density varies depending on temperature; it is generally between 999.7 and 999.9 kg per cubic meter in its liquid form, with a density of between 910 and 960 kg per cubic meter when it is in its solid or gaseous form. These values are influenced as a result of the fact that the metric system of measurement was originally based on a cubic meter of water, due to how much it is found.
As the temperature of earth is, average, approximately 14 degrees celsius, water is typically found in its liquid form. It sees many uses such as as a solvent or to sustain life. It also interacts with and conducts electricity effectively as it is so extremely bipolar. It is typically known to dissolve salt or acids by separating ions in molecules, or to be a catalyst in reactions. For example, water is an effective catalyst in activating enzymes such as the protease bromelain; reactions catalysed by water are known as hydrolytic reactions. Water is both an acid and a base as it self ionises due to its previously mentioned lack of integrity. As it is such a good solvent, and so unique, it is rarely found pure, typically appearing in large concentrations intermixed with other compounds. Overall, water is an extremely critical component in life; after all, we don't drink many liters of it each day for no reason.
Extra reading
For those interested in what lies in their cups or glasses, these sites may be of use:
https://en.m.wikipedia.org/wiki/Properties_of_water
http://witcombe.sbc.edu/water/chemistrystructure.html
http://www1.lsbu.ac.uk/water/water_molecule.html
For those who are questioning the tenacity of my information on the metric system:
https://en.m.wikipedia.org/wiki/History_of_the_metric_system
For information on the different atomic models:
https://www.boundless.com/physics/textbooks/boundless-physics-textbook/atomic-physics-29/the-early-atom-185/the-thomson-model-685-6307/
http://www.kentchemistry.com/links/AtomicStructure/Bohr.htm
Possible investigation could be made into why the atomic bonds between hydrogen and oxygen are so weak in water; whilst they are bipolar, many other substances also exhibit this trait but do not display it so strongly.
Ideas, concepts and difficulties in programming
Whilst the program VRMath is highly effective and simplistic to use, its primitive nature, clunky interface and a lack of guidance resulted in various issues during the process of creating the model of the molecule. As VRMath is based on an old command-based application for creating images, it can only accept specific, simplistic commands. This means that in programming and creating script, only certain commands are accepted by the application, and extremely specific commands must be given to trigger a function. Whilst all programming is like this, coupled with the lack of proper guidance (the crash course at QUT was excellent, but it was still only a crash course) and the counterintuitive nature of some aspects of the logo programmer (interestingly irrelevant name) made creating the model a pain; and this shows in certain aspects of it which could certainly use some ironing down and tweaking. For example, assigning animation to an object or group of objects relative to each other is a pain; you cannot use a script to say move one relative to the other (i.e. RT "obj_0 - "obj1 ROTATE radius 9 4); instead, you must set one as a "parent" object, affecting all other objects on the screen, before assigning movement to other objects. In addition, objects on the overworld andobjects tree do not affect the logo editor. This causes some headaches when you use interface options such as the animator to cause something to happen on the overworld... But have no idea on how to program that... Until you get past the clunky interface and find something to help you do that. This links on to the next issue of the interface being problematic. The interface of VRMath 2 is overly cluttered, with too many windows open and image-based communication, with no text. In addition, some interface options have strange names which do not portray their function. This creates headaches in navigating and using the application; you can't complete anything if you don't know what you're doing. However, despite these drawbacks, VRMath 2 is an interesting application with lots of potential. It also uses unorthodox methods of performing tasks; the setscale, setmat and movement functions are certainly not innovative, however, they make interesting use of number keys and units of measurement. In addition, animation generally uses co-ordinates, and animations involving circles use cycle time instead of degrees per second. VRMath 2 certainly could use some polishing, however, it is definitely a decent program in its own right.
VRMath Program:
https://vrmath2.net/sites/default/files/user/u464/logo/H2O.logoIntroduction
https://vrmath2.net/sites/default/files/user/u464/world/untitled.x3d
Groups: