Sulfur Atom
In this blog I will be discussing the composition, struture and characteristics of the atom sulfur (sulphur).
It is a common atom with many industrial uses, such as the manufacturing of black powder, matches and explosives. It is also used to create rubber, in dyes, and as an insecticide and fungicide, however its main use is in producing sulfuric acid, the top chemical used by the world's industry.
COMPOSITION
With an atomic number of sixteen, sulfur is quite a simple atom, classified as nonmetal. It is composed of the oxides SO2 (sufur dioxide) and SO3 (sulfur trioxide), the hydride H2S (hydrogen sulfide), and the chlorides S2Cl2 (disulfur dichloride) and SCl2 (sulfur dichloride). Sulfur has 18 isotopes, with mass numbers of 27 to 45. Furthermore, 95% of naturally occuring sulfur is in the form of 32S. Its melting point is 388.4K or 115.2C, and its boiling point is 717.9K, or 444.7C.
Sulfur forms many different compounds including the gas hydrogen sulfide, which is known for its strong odour, having been often compared to rotten eggs.
STRUCTURE
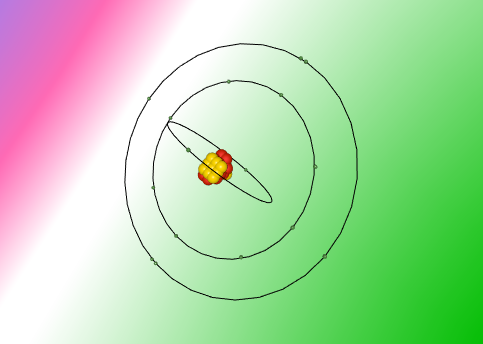
Sulfur is composed of 16 protons, 16 neutrons and 16 electrons. It contains three shells with two electrons being held in the first, eight electrons in the next, and six in the last.
The most significant compound of sulfur used in the industry as previously mentioned is sulfuric acid (H2SO4). Sulfuric acid is a highly corrosive strong mineral acid produced by reacting SO3 with water. Long term exposure to low concentrations or short term exposure to high concentrations can result in adverse health consequences, although it is used to make fertilisers and other chemicals, in petroleum refining, in iron and steel production, as well as many other uses.
CHARACTERISTICS
Being the seventeenth least dense atom, sulfur has a density of 2.07g/cm3 at 293K, an atomic weight of 32.06, and an atomic volume of 15.5cm3/mol. The atom is a soft, pale yellow colour; odourless and brittle in its solid form, thus care must be taken when handling specimens. When kept moist (or not allowed to dry), hyrdogen will mix with the sulfur, forming hydrogen sulfide which causes the deterioration of a specimen. It burns with a blue flame, oxidising to sulfur dioxide, and melting into a molten red liquid.
QUESTIONS I STILL HAVE
Who discovered sulfur? How was it discovered?
Where did it get its name?
More interesting facts?
CHALLENGES
I really had some trouble with the programming. I found that even after following the tutor (Andy)'s instructions, revisiting the manual and using coding examples found on the website by other creators, I still couldn't figure out how to place things where I wanted them to be, and I've heard that many people faced the same challenge. However, I hope that through working together and offering advice and tips, the majority of us have successfully completed our models.
For more information, please visit the following websites:
http://www.lookchem.com/Periodic-Table/Sulfur/#conductivity
http://www.rsc.org/periodic-table/element/16/sulfur
http://www.ducksters.com/science/chemistry/sulfur.php
http://www.livescience.com/28939-sulfur.html
http://www.softschools.com/facts/periodic_table/sulfur_facts/190/
https://vrmath2.net/content/sulfur-atom-grace-dowdle























