Lithium Atom

This blog will discuss the shape and characteristics of lithium atoms. Some questions and will be asked further in the investigation for you to answer yourself.
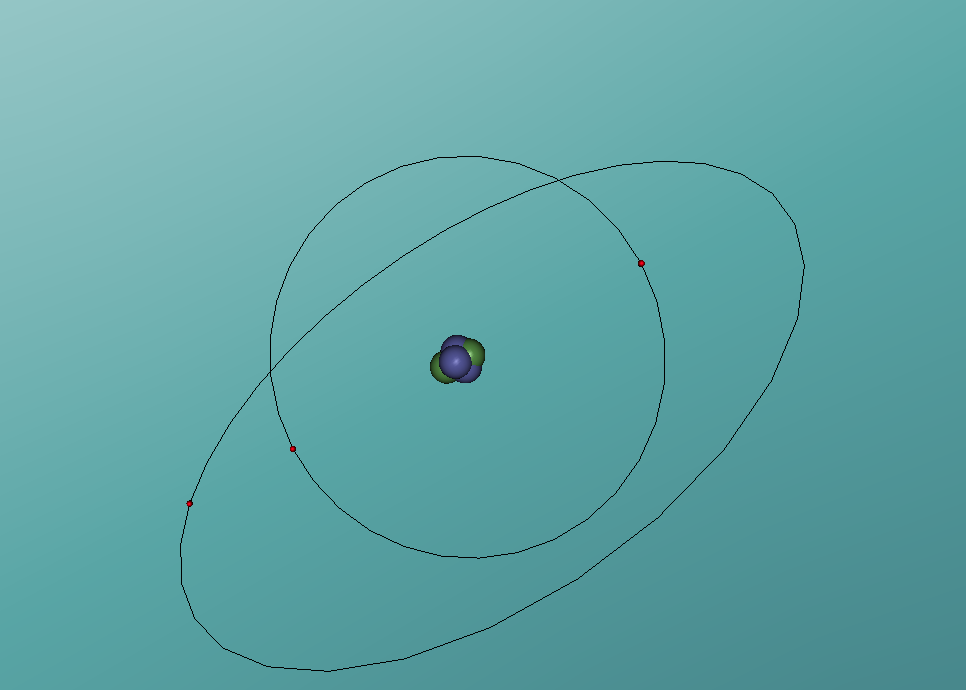
In this model of a 3D lithium atom, it contains features which all atoms have. In the centre of the model there is a nucleus and within it, the neutrons and protons are found. As this is a lithium cell, there are 4 neutrons and 3 protons within the nucleus. Orbiting the nucleus, are the electrons. There are two rings of electrons in the model. The first, most inner ring, contains two 'seats' for electrons, both of which are filled. The second, most outer ring, contains eight 'seats' for electrons yet only one is filled. This is because the second ring will always contain eight 'seats' so no matter how many electrons are needed from an atom in the range of 3-10, there will always be two rings.
Some things that we may want to investigate further are lithium isotopes and how they differ from normal lithium atoms. There are nine variations to the atom lithium, each containing various amounts of neutrons. The isotope with the least amount of neutrons is lithium-4, with one neutron and isotope with the most amount of neutrons is lithium-13, with nine neutrons. Each isotope has one more neutron than the previous one so lithium-5 has two neutrons and lithium-6 has six neutrons. All isotopes except for lithium-6 and lithium-7 can be harmful as they are radioactive while lithium-6 and lithium-7 are stable. Lithium-7 has the same amount of neutrons as a normal lithium atom although the differences between them are that lithium-7 has a slightly higher atomic mass of 7.02 compared to 6.94 and that lithium-7 is slightly radioactive.
There were some difficulties with the programming while making the .x3d file model. Creating electrons that orbited around the nucleus was difficult. We had to put a ball in the middle which is hidden by the nucleus which the electrons orbit around so that electrons orbit at the very centre. Making the rings turn in both the x and y axis was difficult as well as they had to orbit the correct objects in the correct direction. That was the most challenging part of creating the model. The solution to our problem was to simplify the code, allowing us to see what we were doing properly and editing what we needed.
.Logo Code:
clean home reset
setmat 0 21 left 45 fd .3 ball
home bk .5 setmat 0 8 ball
rt 45 fd .5 setmat 0 21 ball
left 135 fd .75 ball
up .25 rt 180 fd .5 left 90 setmat 0 8 ball
dn .5 setmat 0 8 ball
home setscale 1 1 1 setmat 0 8 right 45 fd .3 ball
home setmat 0 35 ball
transform
setparent object
setscale 6 6 6 circle
setscale 10 10 10 circle
spin "obj_8 "tr 10
spin "obj_9 "ru 2
spin "obj_10 "rd 3
right 90 fd 6 setscale .2 .2 .2 setmat 1 0 ball
bk 12 ball
bk 4 ball
home
By Daniel Santic and Sean Leung
Groups:
























