KOH Pottasium Hydroxide Molecule Layke Ellison Final
Potassium Hydroxide is a pretty uncommon chemical around your house compared to H20 (Water), NaCl (Salt) and O2 (Oxygen). However, if you are ever holding a bar of soap, a bottle of hairspray, some fertiliser or even food stabiliser, that may possibly be containing some potassium hydroxide. This blog will include information on the Potassium Hydroxide Molecule, what it contains and how it is structured, the 3D model of the Potassium Hydroxide Model and the issues and questions I had with the whole task.
Potassium Hydroxide also known as caustic potash is made up of Potassium (K), Oxygen (O) and Hydrogen (H). The Hydrogen and the Oxygen (Hydroxide) has a negative charge on the molecule while the Potassium has a positive charge. The total atomic mass of this molecule is 6.1654 moles/g. Potassium Hydroxide is a colourless solid which has a strong base. It has industrial uses because they are able to exploit the corrosiveness and the reaction it has towards acids. The Potassium Hydroxide is used for soap, hairspray and other different household items.
The structure of the molecule involves Potassium, Oxygen and Hydrogen. However, Potassium Hydroxide is also a product of Potassium Chloride and Water combined together:
2KCl + 2 H2O = 2KOH +Cl2 + H2
Potassium Hydroxide physically is a white solid with the density of 2.12 g/mL, with a melting point of 360 degrees Celsius, and a Boiling Point of 1327 degrees Celsius. Chemically, it is a highly hygroscopic solid which can absorb water from the air making it useful as a drying agent. It is very stable in terms of temperature. It can react with acids from a range of potassium salts which are used in the industrial world. Potassium Hydroxide is very corrosive and strong with the ability to penetrate the skin and tissues, and if it lands on your skin or eyes it could cause burns, irritation or blindness. If inhaled, it could damage the mucous membranes and lungs, and if swallowed, it could be extremely dangerous, leading to permanent tissue damage.
Links for more information about Potassium Hydroxide:
http://www.livestrong.com/article/122647-uses-potassium-hydroxide/
The difficulties I had was the website. When we were in the workshop due to an error I could not log on the website for the past hour even though I had already previously registered, and I was a bit behind on the work that everyone else was taught properly on and didn’t miss. I also had to restart the blog 4 times due to different error and bugs which would cause the website to crash or close on its own. I was always curious about the other functions that we were not taught, and I was also intrigued by how we can make further better designs and models of different things for example Pokestops.
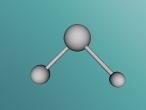
The 3D Model was made up of three spheres and two cylinders. The two cylinders were the chemical bonds which physically held the whole molecule together. The sphere in the middle being the largest was the Potassium atom as it was the largest in its atomic number out of all of them being 19. The second largest one on the left hand side is the Oxygen as it is the second largest atom having an atomic number of 8. Then the third sphere on the right is the Hydrogen atom containing the smallest atomic number of 1.
I hope that this blog could teach you more about Potassium Hydroxide, how it is structured, what it is used for etc. Thank you!