Silicon Atoms
For this project, I decided to simulate a silicon atom using VrMath software. I chose silicon, one of the most abundant elements in the earth’s crust, since it is highly beneficial to human civilisation due to its many electronic applications. Silicon is a metalloid element, a subclass of elements which has properties between metallic and nonmetallic elements, or has a mixture of both. Being in this set, Silicon is metallic in appearance, but is brittle and not highly conductive. It forms a solid at room temperature, with a melting point at 1414 °C, and a boiling point at 3265 °C. As a result of these properties, silicon is widely used as a semiconductor in many electronic appliances. Silicon is used for cosmetic purposes, and is the one of the main components in glass.
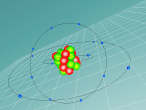
Using the VrMath software, I created a Bohr model of the silicon atom, showing the concentric rings of electrons orbiting a nucleus composed of neutrons and protons. Although the Logo coding program was complex and confusing, I was able to successfully construct a simulated silicon atom after discovering several useful programming techniques.
Silicon atoms are composed of 14 protons (red), and, of course, 14 electrons (blue), since the number of electrons and protons in any atom must be equal, and remain constant, in order for it to maintain a neutral charge and retain stability. However, as neutrons (green) have no charge, they don't affect the atoms charge. Thus, any atom may have more or less neutrons when compared to the number of protons. Adding the number of protons and neutrons designates the exact isotopes of an atom, and there are 24 known isotopes of silicon, with isotopes 28 (14 neutrons), 29 (15 neutrons) and 30 (16 neutrons) being naturally abundant. However, to maintain simplicity, the silicon atom I designed is isotope 28 with14 neutrons. As the atomic weight of an atom is the sum of its protons and neutrons, the standard atomic mass of silicon is 28.085 amu. I also designed the atom so that the electrons were placed in the correct electron configuration. The first shell only contains 2 electrons, while the second contains 8 and the third 4. As the third shell is not filled, the outer four electrons are valence electrons, meaning that the silicon atom will share these four electrons with other atoms in order to appear to only have two complete shells of electrons. As a result of having four valence electrons, silicon atoms tend to form four atomic bonds.
Here are some links to further information regarding silicon:
http://www.lenntech.com/periodic/elements/si.htm
http://www.chemicalelements.com/elements/si.html
http://www.livescience.com/28893-silicon.html
Although initially the construction of the atom was difficult, discovering some useful programming techniques allowed me to more efficiently construct the atom. For example, the construction of the electron shells would have required much repetition was it not for the use of the LOOP FUNCTION. This coding function allowed me to get the turtle to repeat a piece of code a certain number of repetitions. This allowed me to place an electron, then move and place another electron in a different part of the electron shell with only one piece of code. As a result, instead of having to code each electron into its proper place, I only had to code three lines, one for each electron shell, making the construction process of the atom far easier.
Here is the full coding for my Silicon Atom
Groups: