LITHIUM 9D
All the objects that surround us in our lives are made of extremely small particles invisible to the naked eye. They are called atoms and combine to form the materials and substances we need to build objects, eat, drink, breathe, or almost everything we do.
Today we wll discover the structue and features of atoms, using the lithium atom as a simple example. This blog will cover the particles with the atom, its different isotopes and interactions with other atoms.
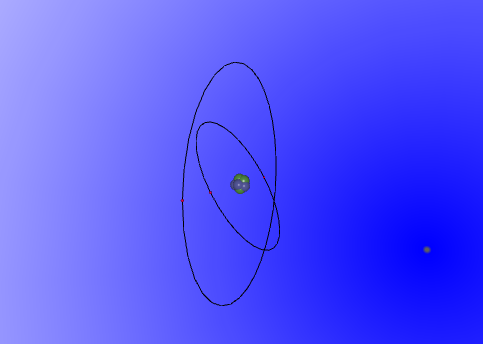
The lithium atom
This image of the lithium model that Daniel Santic and I, Sean Leung have programmed using vrmath2 displays the psrticles of the lithium atom. In the centre there is a cluster of green and blue spheres merged together. This part of the atom is called the nucleus, comprising most of the mass in an atom. The green spheres represents neutrons, which have the most mass compared to the other particles, and does not have a charge. This model contains 4 neutrons. The blue spheres, on the other hand, represent protons, which have slightly less mass than neutrons (the difference is amere 0.3%) and possess a positive charge.There are 3 of these in this model. Orbiting the nucleus are electrons, which are even smaller particles, almost 100 times less in mass than protons and neutrons, with negative charges. They are represented as the barely visible red spheres on the circular tracks, which represent their orbiting route around the nucleus. The reason why the electrons are sperated onto sperate circles of different diameters is because of the capacity of electrons in each of these rings. The most inner ring in all atoms can holda maximum of 2 atoms, the next one outside of it holds 8, the next one holds 18, and the fourth holds 32. There are rings that are larger and hold more electrons, but are less likely to be involved in daily life. The animattion of the model created is available below for thoose who wish to see the orbiting of the electrons.
Atoms have variations called isotopes, which differ in the number of neutrons, and change the characteristics of the atom. The most common isotope of lithium is 7Li, with 4 neutrons, with a natural abundance rate of approximately 95% (Note: the number on the top left of the atomic symbol is the atomic weight, the number of protons and neutrons combined). 6Li, with 3 neutrons, is found in abvout 4% of naturally occurring lithium samples. Both the afforementioned isoyopes are stable and have low nuclear binding energy. However, there are rare instances in which the lithium atoms contain 1, 2, 5 or 6 neutrons, which are unstable and are raioactive, emitting radiation, but often having very short half-lives, 838milliseconds at most.
The way that atoms tend to bond in order to reach a stable state, is very interesting. The electrons of atoms tend to cluster together in attempt to "complete" the electron shells orbiting the nucleus, reaching its most energy-stable state. For example, a lithium will most likely bond with an atom short of one electron on its outmost ring. This causes the atom to reach both atoms to reach their most energy-stable state, and form a molecule at the same time. Atoms with nearly but not yet incomplete electron shells tend to be very unstable and potentially radioactive, decomposing and releasing radiation waves. This effect is larger when atoms with more electron shells are incomplete.
An animation of the 3D lithium atom model.
Attempting to create this model was challenging for both my partner Daniel and I. The coding required to display the electrons and electron shells togther in sync was the most difficult. Another challenge was making the electron shells t rotate in both the x and y axis was difficult to program. The rate and direction of the electron orbit was challenging as well. This was solved when we reffered back to the example model presented by Dr. Yeh, and analysed the coding which enabled different function of the animation, and implementing them into our programminhg in the simplest form.
Program coding on vrmath2:
clean home reset
setmat 0 21 left 45 fd .3 ball
home bk .5 setmat 0 8 ball
rt 45 fd .5 setmat 0 21 ball
left 135 fd .75 ball
up .25 rt 180 fd .5 left 90 setmat 0 8 ball
dn .5 setmat 0 8 ball
home setscale 1 1 1 setmat 0 8 right 45 fd .3 ball
home setmat 0 35 ball
transform
setparent object
setscale 6 6 6 circle
setscale 10 10 10 circle
spin "obj_8 "tr 10
spin "obj_9 "ru 2
spin "obj_10 "rd 3
right 90 fd 6 setscale .2 .2 .2 setmat 1 0 ball
bk 12 ball
bk 4 ball
home
By Daniel Santic and Sean Leung
Further reading on atoms;
Introduction to the atom
https://www.khanacademy.org/science/chemistry/atomic-structure-and-properties
Molecules and compounds
https://www.khanacademy.org/science/chemistry/atomic-structure-and-properties/introduction-to- compounds/a/paul-article-2
The periodic table, electron shells, and orbitals
https://www.khanacademy.org/science/biology/chemistry--of-life/electron-shells-and-orbitals/a/the-periodic- table-electron-shells-and-orbitals-article
Programming:
VRMath 2.0: A Virtual Reality Learning Environment
http://www.x3dom.org/vrmath-2-0-a-virtual-reality-learning-environment/
Groups:























